The human body houses a complex of twisted pathways, labyrinths of tunnels unimaginably small. The biological systems responsible for the flow of the blood, oxygen, and electrical impulses that sustain us are intricate and delicately coordinated. And so, when these systems go wrong, when our bodies are vulnerable to cancers and diseases, it seems at first it would be ideal to have medicine that can perform on a scale as small and complex as the circuitry on which it acts. Rather than exposing the whole body to toxic chemotherapy drugs, imagine cancer treatments that could deliver drugs directly to malignant cells. Consider swallowing a device that could travel through your body, looking for signs of irritation and illness.
Such a world seems surreal and evokes images of science fiction stories and children’s books. However, the possibility of having tiny robots navigate the smallest passages of the human body is not far from being a reality. In fact, important steps have already been taken towards the creation and use of such nanotechnologies. When perfected, these microbots will enable doctors to explore and mend patients’ ailments with greater insight and precision.
First Steps
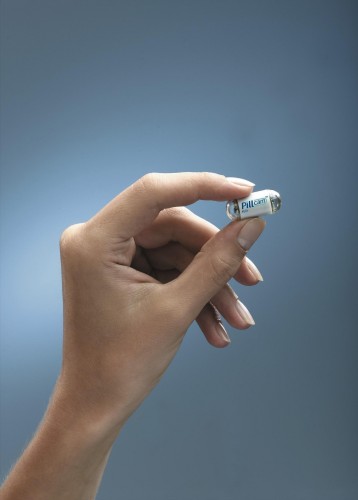
The first step towards using nanotechnology in medicine occurred in 2001, when Given Imaging introduced the PillCam. The PillCam is a capsule containing a light and camera that a patient swallows. Images beamed wirelessly from the capsule can be analyzed and used for diagnostic purposes, thus replacing procedures like the traditional endoscopy, in which a flexible tube containing a flashlight and camera is inserted into the digestive tract. The PillCam, at about the size of a normal pill, is ideal for use in the passageways of the gastrointestinal system since it can be swallowed. However, the digestive system is comprised of relatively large pathways compared to those of the arteries and capillaries, which can be as small as a few micrometers in diameter. The PillCam is thus still too large to travel through the entire circulatory system. Additionally, the device lacks a means of navigating itself through the body; it merely travels passively along the natural course of the digestive system.
Thus, in order to explore passageways like those in the circulatory system, scientists needed to find a means of creating a smaller device that would be able to propel itself against the flow of the bloodstream. The difficulty of this task was largely in the size of the technology needed. Any traditionally built battery-powered motor would be far too large to fit through passages only micrometers thick.
Drug-Delivering Devices
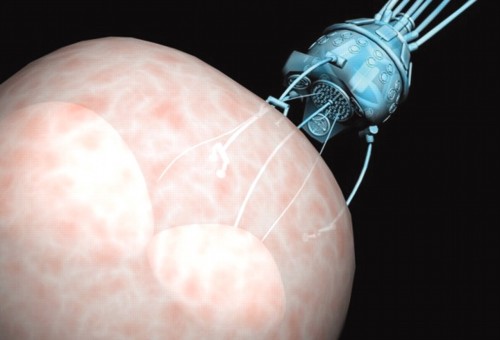
Scientists have managed to overcome this obstacle by using magnets instead of motors to propel the devices. Dr. Sylvain Martel, the founder and director of the NanoRobotics Laboratory at the École Polytechnique de Montréal, and his team have developed microcarriers that are able to pass through the larger arteries. These microcarriers are navigated by the magnetic coils of an MRI machine and have successfully delivered drugs to rabbits’ livers.
Similarly, a team in Dresden has created microtubes made of titanium, iron, and platinum. According to a paper written by this team on their research, these rolled-up microbots are capable of “the selective loading, transportation, and delivery of microscale objects in a fluid.” Like Martel’s technology, external magnets control the motion of these tubes. However, these microbots are also propelled by microbubbles. The tubes are partially filled with hydrogen peroxide, which, in a reaction catalyzed by the platinum, decomposes into oxygen and water. The force of the bubbles ejected from the tube during this reaction propels the microtubes through the body’s passageways. Additionally, the diameter of these tubes is at around five micrometers, about one-tenth the size of the microcarriers utilized by Martel’s team, thus enabling them to transverse much smaller arteries.
According to an article written by Martel, “the [technology’s] first real application will be in treating cancers.” These drug-delivering microbots are preferable to current means of fighting cancer because they can bring the medicine directly to the tumor, helping to avoid killing healthy cells along with the cancerous ones.
Bacterial Microcarriers
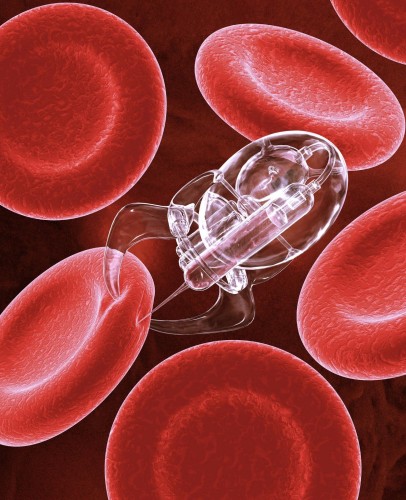
Another solution to the problem of size can be found in nature. The MC-1 strain of bacteria, discovered in 1993, is magnetic and propels itself with spinning tails. This strain is ideal for use as microcarriers of drugs because, at 2 micrometers in diameter, it is small enough to navigate even our bodies’ tiniest capillaries and can be controlled by use of a relatively weak magnetic field. Martel’s team has already tested this system on mice, guiding a swarm of drug-carrying bacteria to tumors in the animals’ bodies. A team at Purdue University has performed similar experiments in mice, adhering genes to the surface of bacteria to alter gene expression in cells. In test cases, mice were injected with bacteria carrying genes for luminescence. The scientists found that certain organs in the animals’ bodies successfully expressed the luminescent genes, suggesting that these bacteria could be used for altering the gene expression in diseased cells.
Though this bacteria-driven technique does navigate through more of the human body than its man-made counterpart, this method is not without faults. In Martel’s experiments, for example, most of the bacteria never reached the tumors. The bacteria has a half-life of about 30 or 40 minutes, so many of the bacteria died before reaching the tumor. Additionally, many were misdirected by the strong currents in the animals’ larger vessels. Martel offers a hybrid solution to this problem. His team is currently pursuing the possibility of using man-made microbots to transport the drug-carrying bacteria through the larger vessels to get closer to the tumors. Then, when the microbots are unable to go farther through the small vessels, they would release the bacteria. Since, in this scenario, the bacteria would be dispatched closer to the target, the hope is that there would be a greater likelihood in reaching the tumor.
Diabetes Regulation
In addition to cancer treatment, microbots are also being considered potentially useful for other medical purposes. For example, a team in Australia has proposed a concept and simulation using nanobots to regulate diabetes. Diabetes patients have to test their blood multiple times daily to ensure that their glucose levels are stable. The Australian team proposes using nanorobots to travel through patients’ bloodstreams and send data about glucose levels to external electronic sources. Using nanorobots would enable doctors to receive data from many different locations simultaneously throughout the body and allow for a more continuous monitoring of blood sugar levels without the pain and inconvenience of self-testing. Additionally, unlike the technologies explored for drug-delivery, these nanobots would not require active motion. Rather, they could travel with the natural flow of the bloodstream, sensing blood sugar levels along the way. Their passive movement makes it much easier for scientists to design these structures, which do not have to include any means of propelling or navigating themselves through the circulatory system. Like the proposals for cancer treatment, these nanotechnologies afford a more convenient and precise methodology for diabetes regulation.
Looking Ahead
Though these microscopic methods of treatment and diagnostic tests are not yet perfected, scientists are getting closer to realizing a world of medicine that is able to navigate the minute scale of our bodies’ smallest pathways. Concepts of and experiments on medical nanotechnology are presenting doctors with new potential for treating their patients precisely and conveniently. As Martel notes in an article in IEEE Magazine, “There is no shortage of possibilities… a real-life fantastic voyage is just beginning.”
