The origin of epithelial ovarian cancer is not the ovary, according to a recent discovery in the lab of Dr. Gil Mor. Recent results show that multiple peritoneal organs and cell types are involved in the initiation of this mysterious cancer. His results help explain the high recurrence rate of epithelial ovarian cancer; 60 to 80 percent of patients who initially respond to chemotherapy experience recurrence within two years, post-treatment.
Mor’s research addresses one problematic aspect of the classic ovarian cancer model: the ovarian tumor as a set of homogeneous cancer cells. Discoveries within his lab directly address this misconception, and together postulate a new model for the origin of ovarian cancer.
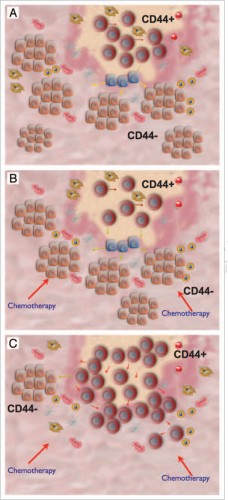
In 2005, the Mor Lab, using the stem cell markers CD44 and MyD88, identified two distinct cells types within the ovarian tumor: chemo-resistant Type I cells, characterized by slow growth and stem-like properties, and chemo-sensitive Type II cells, the “classic” cancer cell characterized by fast growth.
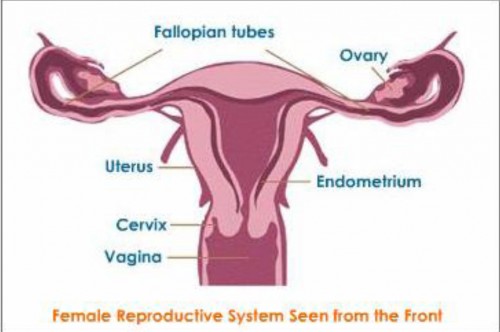
But these two cell types are not the only contributor to the heterogeneity of the ovarian tumor. There are multiple types of ovarian cancer with characteristics that are not from ovarian cells but from the cells of other organs in the female reproductive tract, such as the fallopian tubes, uterus, cervix and other peritoneal organs. This raises several questions. Why is the ovarian tumor comprised of such a diverse array of cell types? Where do those cells originate, if not from the ovaries? How do these cells migrate to the ovary? And ultimately, how does knowledge of these mechanisms translate into new therapies to combat ovarian cancer?
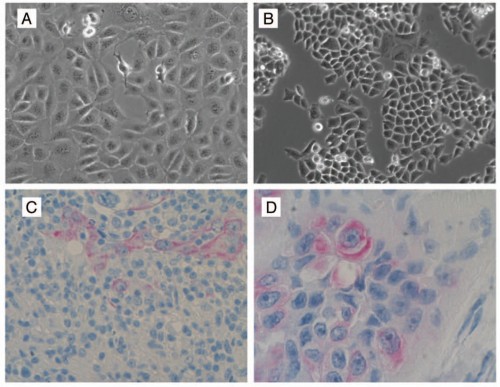
The Mor Lab has linked the epithelial to mesenchymal transition (EMT) with the migration of these heterogeneous cell types to the ovary. Through this process, Type I epithelial cancer cells gain the ability to migrate through the female reproductive tract by turning into mesenchymal cancer cells. The reverse process, mesenchymal to epithelial transition (MET), causes these migratory cancer cells to attach to the ovary and revert to a fast-dividing Type II cancer cell phenotype.
To confirm this hypothesis, human ovarian Type I cancer cells were injected into the uterus or the peritoneum of cancer-free mice. Through life imaging techniques, the Type I cells were found to migrate toward the mouse ovaries. Once the cells reached the ovaries, these cells then transitioned to epithelial cancer cells through MET and formed ovarian tumors, characteristic of ovarian cancer tumors seen in patients.
This model elucidates the mechanism through which cells from varying sites of origin migrate to the ovary, creating histologically diverse tumors. It also illustrates the important role of the stem-like Type I cells in initiating epithelial ovarian cancer. The heterogeneity of the ovarian tumor caused by the Type I epithelial ovarian cancer cell indicates the need for novel treatment methods geared toward eradicating this specific cell type.
