Imagine never having to worry about replacing the batteries in your electronic devices. Such technology may not be far from our grasp. André D. Taylor, Assistant Professor of Chemical and Environmental Engineering at Yale, is the principal investigator on the cutting edge of a new micro fuel cell technology that may serve as a viable alternative to the batteries utilized in a vast array of portable electronic devices. Composed of bulk metallic glasses (BMGs), these novel fuel cells have the potential to be inexpensive, long-lasting, and environmentally friendly.
BMGs are a type of metal alloy that demonstrate a unique characteristic: Instead of the crystalline structure associated with most metals, these BMGs have random atom placement. For this reason, BMGs are highly malleable, while still possessing the strength that accompanies metals. Specifically, the micro fuel cell components developed by Yale researchers are composed of platinum and zirconium alloys.
In general, fuel cells are electrochemical conversion devices that transform various fuel sources — such as hydrogen gas or hydrocarbons — into electricity. Unlike batteries, these cells can continuously supply electricity as long as a fuel is supplied.
In contrast, batteries, which are electrochemical storage devices, rely on a distribution of ions. Potential energy resides within the battery, where positive and negative ions are separated, creating an electrically neutral situation. In a fuel cell, the oxidizing agent and fuel source are separated by a membrane that does not allow for direct transfer of electrons. For this reason, electrons must travel a circuitous path to reach the endpoint of the reaction, and it is along this path that energy is collected. In most cases, while this electron transfer is harvested as electricity, water and carbon dioxide are produced as byproducts of the oxidizing reaction. The biggest difference between these two energy sources is that a battery has a limited lifetime and a diminishing energy output, whereas a fuel cell has a steady energy yield.
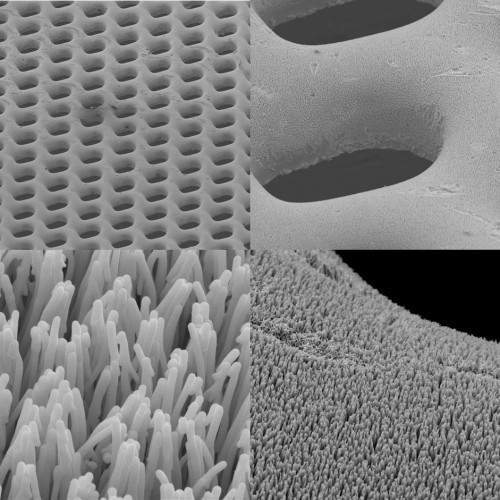
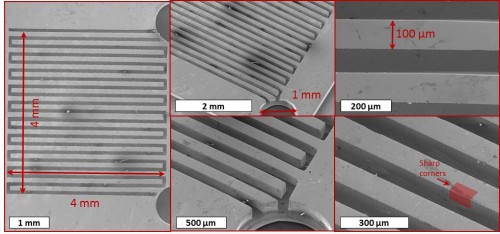
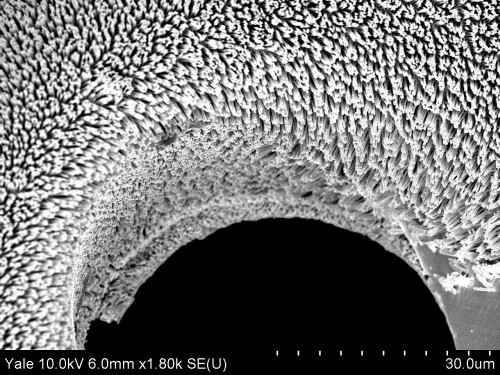
As their name suggests, micro fuel cells are much smaller than the average fuel cell, with dimensions of approximately 0.16 square millimeters. Generally, such cells are produced with either silicon or stainless steel; however, silicon conducts electricity poorly and is brittle, while stainless steel cells corrode easily. For this reason, BMGs are favored. With high corrosion resistance and great pliability, these conductors are more suitable for use in portable electronics. In addition, BMGs are far less expensive to fabricate than their silicon and stainless steel counterparts, since the latter require special coatings to circumvent corrosion and brittleness.
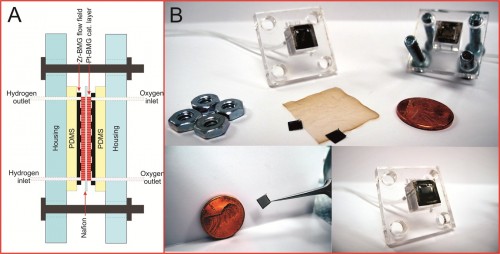
The micro fuel cell that Yale researchers in the Taylor Lab produced is a micro-fabricated device, or a device consisting of miniature structures. Its construction comprises four layers of BMGs, which are based upon zirconium and platinum. The platinum BMGs catalyze the oxidation, whereas the zirconium BMGs provide the medium through which the electrons travel. The polymer separating the two layers of BMGs is called Nafion, which is an ion-exchange material that is effectively permeable to protons. In addition, researchers generated another micro fuel cell with a more typical platinum-carbon (Pt/C) layer replacing the platinum BMGs. With the platinum BMG cell, the maximum power output recorded was smaller than that recorded for the cell prepared using the Pt/C electrode. Even so, the latter recorded value was a marked improvement over the previous best power density in existing literature, further demonstrating the viability of BMG micro fuel cells. In the near future, these cells may be utilized in various portable electronic devices, such as smart phones, tablets, and sensors. This inexpensive and eco-friendly fuel cell technology may have the potential to become a common feature in our lives, since it can be used in such a vast assortment of electronics.
As principal investigator for this project, Taylor adds that “Our research is a demonstration showing the versatility of these materials. In the future, we hope that these micro fuel cells will replace the standard lithium ion battery.” Currently, the team is working to increase the amount of power output generated by the micro fuel cell. Their research has been published in the journal Small, and Ryan C. Sekol, a doctoral candidate in the Taylor Laboratory, is lead author. The project was performed in collaboration with Jan Schroers, a Professor of Mechanical Engineering and Materials Science, and his research group.
