Imagine having the power to create drugs by picking and choosing substituents and fitting them together like the pieces of a model. Not only would existing drugs become easier to produce and access, but countless new pharmacologically active compounds would become available to treat disease. Exciting research recently published by Yale Professor Jonathan Ellman, Eugene Higgins Professor of Chemistry, suggests that this goal may not be so farfetched.
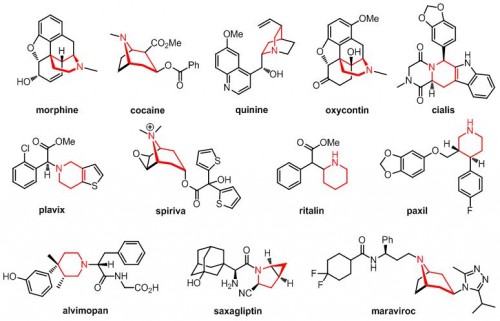
Ellman’s study, published in Science, involved the addition of pharmacologically active substituents to molecules called piperidines. These molecules are already pervasive in drug syntheses and serve as the center of several widely used pharmaceuticals including morphine, Plavix, Cialis, and Ritalin. Piperidines have hexagonal structures with several sites open to the addition of functional groups, the reactive building blocks of organic molecules. This trait gives them great synthetic potential, but poses a problem for control of stereochemistry, or the arrangement of substituents in space. A slightly different conformation, or stereoisomer, of a drug can have strikingly different and even harmful effects. Thalidomide, for example, was marketed in the late 1950s as a cure for morning sickness, but was quickly withdrawn after scientists linked a rise in birth defects to the teratogenic effects of its stereoisomer.
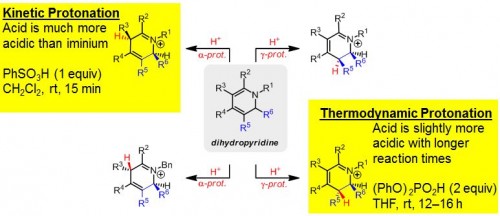
“There are many ways you could place functionality onto piperidines and, if the process was non-selective, you’d have more than eight possible products, which would just be a disaster,” said Ellman. Drug syntheses need a single pure product to avoid introducing harmful stereoisomers into the body.
Ellman’s lab has shown that stereochemistry can be precisely controlled through alterations in acidity to specify the sites of protonation on piperidines. A strong acid can force creation of the kinetic product, with protons bonding to the site of highest electron density. A weak acid cannot keep protons on this site and results in the lower-energy thermodynamic product and alternate bonding. The products created in this way have precise, predictable stereochemistry that guarantees the synthesis of a pure compound.
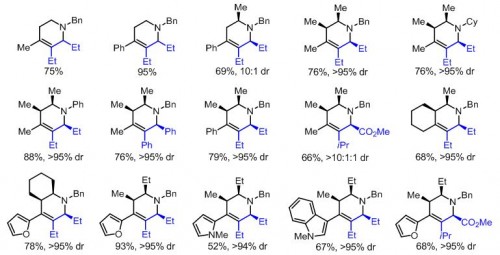
These results suggest a new paradigm in drug design. Professor Ellman’s method requires only readily available materials and relatively simple alterations in acidity to proceed, and yet opens the door to the development of hundreds of potential drugs. Pharmaceutical companies will undoubtedly find the research invaluable both for creating new drug candidates and improving the syntheses for existing piperidine derivatives. Ellman has even published his paper without patent constraints to this end.
“That’s our approach,” he says. “We just want to get it out there and, if it’s good stuff, people are going to use it.”
With further development, this research could have widespread effects on the design and synthesis of drugs. “Of course,” Ellman remarked, “design is important, but not sufficient. We need to figure out which targets are the right targets to hit.”
It would appear a significant amount of work is still required before drugs for any and all diseases can be synthesized. However, if biology can offer up the required targets, Ellman’s chemistry will help pave the way for a revolution in drug design.
