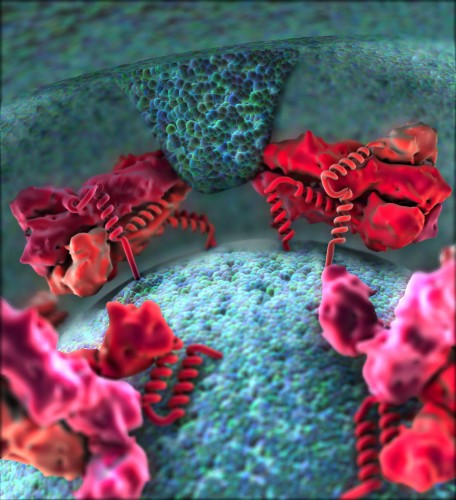
Dengue, caused by a mosquito-borne virus that infects 100 million people each year, is becoming a global health concern. According to the World Health Organization, half of the world’s population is at risk. Marked by high fevers and vomiting, Dengue, though usually non-life-threatening, can turn into the deadly Dengue hemorrhagic fever.
The Dengue virus belongs to a genus called Flavivirus. All flaviviruses have similar infection pathways. Under the leadership of Associate Professor of Molecular Biophysics and Biochemistry Yorgo Modis, Yale researchers at the Richards Center for Structural Biology have recently conducted a study on two other flaviviruses, Yellow fever and Japanese encephalitis, to shed light on the Dengue virus’s infection pathway.
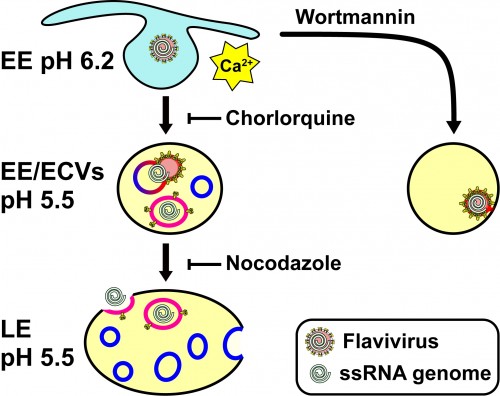
“By inhibiting certain steps of the infection pathway and then testing virus infectivity, we saw how flaviviruses infected a cell,” Modis said.
Researchers previously knew that flaviviruses enter the cell through the endocytic pathway (the cell’s transport system), but not how they get their RNA close to the nucleus, which is key to maximizing infectivity. The new study shows how this is achieved: the virus fuses with a vesicle within the endosome, releasing its RNA into it. Then the virus waits as the endosome follows regular cell protocol—the endocytic pathway—and releases its RNA near the center of the cell, next to the nucleus.

Understanding the virus’ infection pathway is not necessary to creating a vaccine against Dengue. But, Modis explained, “Vaccines can only be pre-emptive. Knowing how these viruses work allows us to create therapeutic drugs for those needing acute treatment.”
