
Introduction
In the early 1900s, Alois Alzheimer analyzed the nervous tissue of one of his most intriguing patients: a woman who had experienced significant memory loss. At the time Alzheimer had no idea of any definitive diagnosis, and when he autopsied her nervous tissue upon her death, he found an anomaly. Alzheimer observed atrophied gray matter around the entire brain, and bundles of neurofibers and plaques. Though his discovery initially went unnoticed, with time, the scientific community realized Alzheimer had unraveled one of medicine’s most mysterious puzzles. He had also raised an entirely new set of questions that researchers are still attempting to answer.
Recently, a team of researchers led by Dr. Stephan Strittmatter at the Yale School of Medicine has come to the forefront of answering some of the mechanistic questions underlying the disease. The team has elucidated a key step in the Alzheimer’s disease pathway — a discovery with revolutionary implications for drug development. Still, some of Alois Alzheimer’s initial questions have yet to be answered.
What is Alzheimer’s?
Normally, Alzheimer’s disease is associated with forgetfulness. Beginning with minute day-to-day details, patients eventually forget even the family members that are nearest to them. This forgetfulness is characteristic of dementia, and Alzheimer’s disease is the most common type of dementia, accounting for about 70 percent of all cases. While dementia manifests itself primarily with age, it also may be genetic or acquired: both cases have been observed.
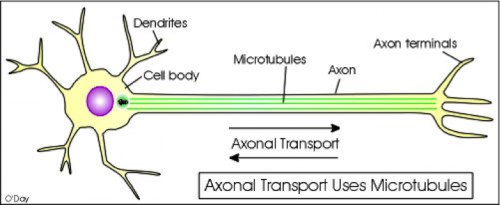
Physiologically, dementia results from the formation neurofibrillary tangles from hyperphosphorylated tau proteins in neurons. When the hyperphosphorylated tau forms, it aggregates near microtubules. Kinesins, molecular motors that transport vesicles away from the synapse, travel on microtubules “like a railroad,” Strittmatter described. When neurofibrillary tangles aggregate along the microtubule, they impair the transportation mechanism of the axon. In other words, neurofibrillary tangles create roadblocks for travelling kinesins. This results in the impairment of neuronal synapses, and thus memory loss, in dementia patients.
Alzheimer’s disease involves seven separate stages, ranging from no impairment to very severe decline. Initially, the disease was only characterized in post mortem cases in which the patients had blackened areas of dead neurons. Today, scientists know that this increased darkening is caused by the presence of increased amyloid beta (AB) plaques and hyperphosphorylated tau protein. But what causes these neuropathologies?
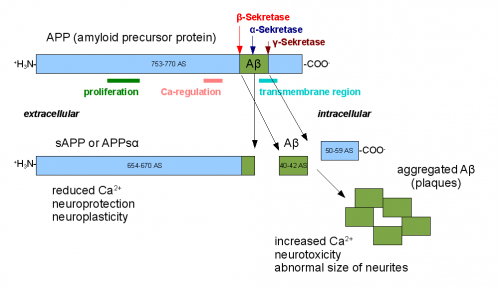
AB plaques are made of AB protein, which is actually found naturally in the human body. Normally, the protein forms when Amyloid beta precursor protein (APP) is cut by the enzyme alpha-, followed by gamma-, secretase. If there is a mutation in the presenilin 1 gene, the AB protein is cut incorrectly. These incorrectly cut AB can aggregate and form amyloid beta plaques.

When APP cuts the original AB incorrectly, an insoluble form of amyloid beta protein results. This insoluble form aggregates into oligomers, which can then form AB plaques. These plaques distinguish Alzheimer’s disease from other forms of dementia, and their formation can also trigger increased phosphorylation (hyperphosphorylation) of the tau protein. Tau protein is normally found in neuronal cells to stabilize microtubules; however, when tau is hyperphosphorylated, it forms the neurofibrillary tangles, which further impair the memory of Alzheimer’s patients.
These neurofibrillary tangles and AB plaques cause the deterioration of and reduction in the number of synapses in the brain of an Alzheimer’s patient. Thus, the insoluble AB plaques disrupt neuronal function based on their concentration, structure and length. The AB plaques interact with membrane surface proteins, gangliosides, and calcium permeable neurotransmitter mediated receptor channels. When these AB plaques interact with those proteins in the cell membrane, neuronal activity and synaptic function is impaired. Specifically, it results not only in synaptic malfunction, but also the loss of dendritic spines.
These membrane proteins are “docking sites” for proteins that activate dangerous signaling pathways. One such membrane surface protein is the prion protein. Mutations in the prion protein can result in neuropathological disorders such as mad cow disease, but this protein also serves as a “docking site” for the amyloid beta protein in the case of Alzheimer’s.
When AB levels increase, more AB binds to the prion protein. This results in overexpression of the Fyn kinase mediated pathway and impairs synaptic activity, which manifests as Alzheimer’s disease. Because the extracellular A-beta plus prion protein complex is involved in intracellular signaling, researchers knew the prion protein complex must have been linked to a signaling hub that mediated the transfer of signal from the extracellular protein complex to the nucleus. This signaling hub is known as a G-protein coupled receptor.

The Strittmatter Lab: Forerunners in Novel Techniques
The G-protein coupled receptor is where the work of the Strittmatter Lab comes in. The team of researchers has recently discovered the identity of the signaling hub: mGluR5, a metabotropic glutamate receptor. This receptor contains seven transmembrane spanning alpha helices and is a G-coupled protein receptor. In other words, when the neurotransmitter glutamate binds to the receptor, it activates many downstream signaling cascades essential for proper cellular function. Normally, the glutamate is responsible for calcium release pathways and src homology signaling cascades. It also activates the Fyn kinase pathway responsible for downstream phosphorylation, and other similar pathways such as the MAPK pathway, JNK pathway, and Cdk5 pathway.
However, when amyloid beta oligomers bind to the prion protein, the mgluR5’s function is altered. In fact, as Strittmatter emphasized, “the A-beta causes the mGluR5 receptor to signal a lot more to downstream pathways, leading to chaos and confusion in the cell.” In this way, the balance of pathways to which the mGluR5 receptor signals is skewed tremendously over time. As the concentration A-beta oligomer increases, it is more likely to bind to the prion protein and overstimulate downstream pathways. Overtime, this desensitizes the mGluR5 such that it does not even respond to glutamate binding. This impairment of mGluR5 activity impairs the postsynaptic function of the neuron, resulting in destruction of synapses and neuronal deficiency: otherwise known as Alzheimer’s disease.

Strittmatter’s research is a major contributor to the recent push for new methods of drug development for Alzheimer’s disease. Most drugs currently target the gamma and beta secretase, however this results in impairing other functions of the cell. Similarly, drugs targeting the AB plaques are only useful if the Alzheimer’s is caught early on; otherwise, the AB plaques have already developed and cannot necessarily be degraded.
Though many drugs attempt to attack a disease without analyzing the possible implications of their treatment, Strittmatter’s research gives drug companies a specific target. Strittmatter mentioned, “drug discovery should occur in parallel with laboratory research; as soon as a new pathway mechanism is discovered, drug companies should see if it can be a potential target for drugs.” As the cofounder of Axerion Therapeautics, he is in the ideal position to “make these parallel discoveries and translate scientific advancements into clinical trials.”
What next? Future Implications of the Research
Immunotherapy techniques that would utilize antibodies to target the a-beta binding site on the prion protein are currently being researched. Strittmatter is particularly interested in targeting the mGluR5 receptor and prion protein with drugs, as these two components are in the “middle” of the pathway to Alzheimer’s disease. What is particularly interesting about the Strittmatter lab’s discovery is that for the first time, drugs can be developed that do not target the beginning of the Alzheimer’s cycle (the AB plaques), or the last component of the cycle (the neurofibrillary tangles). Rather, because the missing middle link in the Alzheimer’s pathway has been discovered, Strittmatter and Axerion Therapeutics, have a novel target in mind with their small molecule approach. “Never before has an emphasis been placed on this new approach midway through the Alzheimer’s pathway; we’re not going as far downstream as tau,” said Strittmatter. Instead, they are targeting the middle men: the prion protein and the mGluR5.
About the Author
Naaman is a junior in Morse College, and is also the Outreach Chair for YSM. She works in the Horvath Lab of Comparative Medicine in addition to doing research at the School of Public Health.
Further Reading
1. “Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron (2013): 887-902, accessed October 22, 2013.
2. “The role of metabotropic glutamate receptors in Alzheimer’s disease. Acta Neurobiol Exp (Wars) (2004): 89-98., accessed October 23, 2013.
