Treating cystic fibrosis today can feel like an infinite game of whack-a-mole. The doctor must manage various symptoms in the lungs and digestive system, all of which require constant attention. Aggressive treatments can lead to severe side effects. Fortunately, a team led by Dr. Emanuela Bruscia, assistant professor of pediatrics at the Yale School of Medicine, has identified a potential new treatment targeting the inflammatory response of cystic fibrosis that could improve quality of life for many patients.
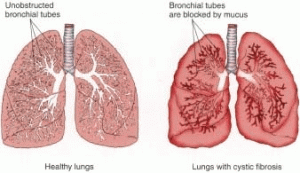
Primarily considered an epithelial tissue disorder, cystic fibrosis causes tissue in the digestive and respiratory tracts to secrete a thick mucus that interferes with digestion and breathing, respectively. The mucus in healthy lungs washes out the pathogens that we inhale, but the buildup of mucus for a cystic fibrosis patient leads to frequent lung infections. These infections contribute to excessive inflammation in the lungs, which over time damages the tissue and hinders lung function. Recent research suggests that the mutation that causes cystic fibrosis may also cause the immune system to overreact to pathogens. Together, the infections and overactive immune system result in excessive inflammation of the lungs.
In collaboration with the directors of the Yale Cystic Fibrosis Clinic, Drs. Marie Egan and Jonathan Koff, scientists in Bruscia’s lab investigated the receptors involved in the innate immune response to find a more efficient means of lowering their activity in cystic fibrosis patients. Specifically, they analyzed the levels of various immune signaling molecules. They found that the COX-2 inflammatory pathway was highly responsive in CF mice, or mice with a mutation analogous to the human cystic fibrosis mutation.
With the help of Yale geneticist Dr. Jun Lu, the team found that CF mice had unusually high levels of a particular microRNA, miRNA-199a-5p. Unlike most RNA, miRNA does not encode a protein, but can regulate the production of proteins. In this case, miRNA-199a-5p should have been downregulated as part of the inflammatory response, but was instead present at higher levels in the CF mice. Ultimately, the incorrect regulation resulted in a lack of negative feedback for the inflammatory response, causing excess respiratory inflammation.
The dysfunctional pathway explains why ibuprofen has been found to lower lung inflammation in cystic fibrosis patients. However, the necessary dosage is so high that many patients cannot tolerate the side effects. Ibuprofen acts by blocking the same COX-2 pathway that Bruscia’s team has been studying. However, since the problems arise upstream in the signaling pathway, Bruscia’s group found a possibly more efficient treatment. Celecoxib, also called Celebrex, is an FDA-approved drug for treating arthritis inflammation. It works by stimulating the downregulation of the same miRNA found at high levels in the CF mice. Since celecoxib acts further upstream in the cascade than does ibuprofen, lower doses of celecoxib are needed for the same effect as ibuprofen therapy. In fact, Bruscia’s lab found that celecoxib worked at one fourth the necessary dosage of ibuprofen. This difference could greatly reduce the experience of side effects for cystic fibrosis patients who need anti-inflammation treatment.
However, before cystic fibrosis patients can take advantage of this new treatment, more research has to be done. Bruscia’s future studies will focus on developing a more detailed picture of the dysfunctional inflammatory response in cystic fibrosis. Egan pointed out that although Celebrex is safely on the market for arthritis, it is not yet FDA-approved for cystic fibrosis treatme
nt, which is a necessary step before the medication can be broadly disseminated. She hopes to advance a clinical trial for the use of Celebrex in human cystic fibrosis patients. “If you want to do it systematically, the next step would be to approach the company that owns the patent and set up a clinical trial,” Egan said.
The clinical trial process is much faster with a drug that is already FDA-approved for another disease — Celebrex has already jumped the hurdle by proving to be safe for use in humans. Hopefully, this means that the drug may soon be able to help cystic fibrosis patients worldwide.
