Whether in the form of a stealth-cloaked aircraft or a disappearing gadget, invisible electronics are a favorite concept in the minds of sci-fi enthusiasts and futurists alike. With recent technological progress, these invisible gadgets may soon become more than the stuff of movies.
Ongoing research has advanced transparent technology in the realm of car dashboards, windows, and personal wearable devices. However, one stumbling block remains: the challenge of making transparent batteries. Yale researchers led by professor André Taylor, director of the Transformative Materials and Devices Lab, are using nanotechnology and a unique method of laying down transparent material to meet this challenge. Their findings are detailed in a paper published in ACS Nano, and provide a promising venture into a world with truly invisible electronics.
In developing invisible electronics, scientists have explored a variety of techniques. A few well-known methods include projecting images or shrinking the size of device components to below the optical threshold. Transparent conductive materials (TCMs), which are already found on appliances such as touchscreen displays, have been critical for these methods. Although TCMs come in a variety of forms, from metal oxides to carbon nanotubes, their transparency often comes at a steep tradeoff with conductivity. Unfortunately, the problem is only exacerbated as the total amount of material is reduced. Batteries must understandably prioritize energy storage, and therefore, they have traditionally required relatively large electrodes made of visible materials.
To address this limitation, Taylor’s team developed a method of creating ultra-thin carbon nanotube electrodes that combine conductivity with transparency. The key lies in a technique called spin-spray layer by layer (SSLbL) assembly. SSLbL assembly sprays alternating solutions of nanotubes, charged ions, and rinses onto a rotating scaffold or platform. As the solutions dry and charges interact, the nanotubes arrange into networks of ultra-thin films, which can be used as electrodes or other devices.
“The needs of [transparent] devices demand processes that have just the sorts of benefits offered by SSLbL — namely, precision and control,” said Forrest Gittleson, lead author of the paper. Indeed, SSLbL can be used to fine tune properties such as conductance and thickness in a way that would otherwise be difficult at a microscopic level.
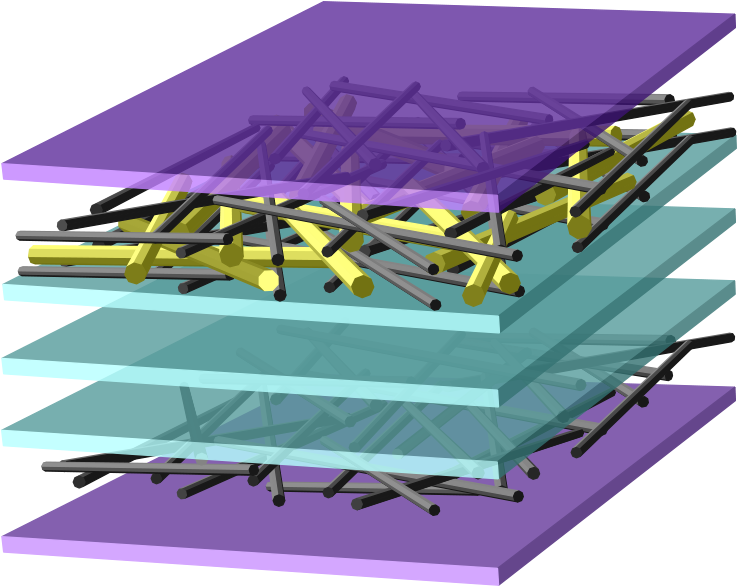
Using single-walled carbon nanotubes and vanadium oxide nanowires as TCMs for the films, the Yale scientists used SSLbL assembly to make lithium-ion electrodes, commonplace in commercial batteries. The new nanomaterial electrode is more than 100 times thinner than typical battery electrodes, and most importantly, is transparent. A sandwich design placing the nanowires between layers of nanotubes combines the properties of these two materials to improve overall conductivity.
In short, this research takes a pioneering step on the road towards transparent batteries, and by extension, invisible electronics.
“We took it as a challenge to take up the last element that others couldn’t make invisible. The research creates a pathway that shows it can be done,” Taylor said.
Nonetheless, both Taylor and Gittleson recognize that much work is ahead. Future directions of research will involve extending the SSLbL method to the other components of a battery as well as improving conductivity and stability.
The costly energy needs of most electronics today will certainly call for even more inventive approaches to transparent batteries. Until then, we can all still enjoy the cinema’s visions of what soon could be.
Cover image: A schematic of an SSLbL-assembled battery shows sandwiched layers of nanotubes and nanowires. Image courtesy of Forrest Gittleson.