In the summer of 2015, an alarming wave of mothers delivering babies with small, abnormal heads hit Brazil’s medical wards. Doctors were baffled. Then, a few officials noticed that the outbreak coincided with a rise in Zika virus infections. The urgency of this medical mystery prompted the World Health Organization to declare a public health emergency. Pregnant mothers were warned not to travel to tropical regions, and news outlets began declaring an “epidemic of birth defects” that threatened the globe. As Brazil prepared for its Summer Olympic Games, Zika virus stole the spotlight as the biggest epidemic since the 2014 Ebola outbreak.
By 2016, the birth condition known as microcephaly was labeled as “congenital Zika syndrome.” The rare condition results from insufficient brain growth in the developing fetus, which causes the baby to be born with a small head without a forehead. Zika is a virus related to West Nile, Dengue, and yellow fever and is commonly found in tropical, equatorial regions of the globe. The virus spreads via a mosquito vector, most commonly the female Aedes aegypti mosquito. Although multiple case studies pointed to Zika as the culprit for the rise in microcephalic births, there was little hard evidence to connect the virus to the disease.
A collaborative effort
A team of researchers at the Yale School of Medicine established some of the first fundamental links between Zika virus infection and microcephaly. Drs. Nenad Sestan, Tamas Horvath, and Brett Lindenbach, as well as their colleagues from the Departments of Neuroscience and Microbial Pathogenesis, came together and combined their expertise to uncover some of the molecular mechanisms behind congenital Zika syndrome.

Through carefully conducted experiments, the researchers demonstrated that the Zika virus preferentially infects neuroepithelial stem (NES) cells, the earliest line of developing neurons. The virus also inhibits cell division in the developing brain by redirecting crucial enzymes to incorrect targets during mitosis. In addition, the team stumbled upon a potential antiviral drug that inhibits Zika virus replication. Remarkably, the team of researchers unveiled not only crucial molecular pathways necessary for early brain development, but also a possible path to treatment.
The study and its results came together rapidly, advancing from conception to publication in a matter of months. “It was very exciting,” Sestan said. “Here was something that posed an immediate biomedical threat to society, and whoever we called for advice was already thinking, or doing something, about it.”
Sestan and his colleagues had been studying NES cells in their lab for many years. Meanwhile, Lindenbach’s lab, located on the same floor in an adjacent building on Yale’s medical campus, studied the replication of positive-sense RNA stranded-viruses, a class of viruses that includes Zika virus. Recognizing that the emergence of Zika virus raised multifaceted questions spanning multiple fields, the two labs teamed up to contribute their respective expertise to an investigation of the mysterious Zika virus and how it led to microcephalic babies.
“We were just socializing before we realized, ‘Wow, maybe we can work together on this interesting problem,’” Sestan said.
A molecular understanding of Zika
The first half of their research, published in Cell Reports, addressed Zika virus’ capability to infect neuronal stem cells. The researchers showed that Zika virus preferentially infects both NES cells and radial glial cells, important cells that provide structure for the developing brain. The team replicated their findings in both mouse and human cell cultures. Infected radial glial cells were also detected in tissue samples obtained from prenatal microcephalic brain tissue in a Zika-infected mother. This critical anomaly in growth not only resulted in cell death, but also led to an “architectural disruption” of the glial cell scaffold that is necessary for proper brain development.
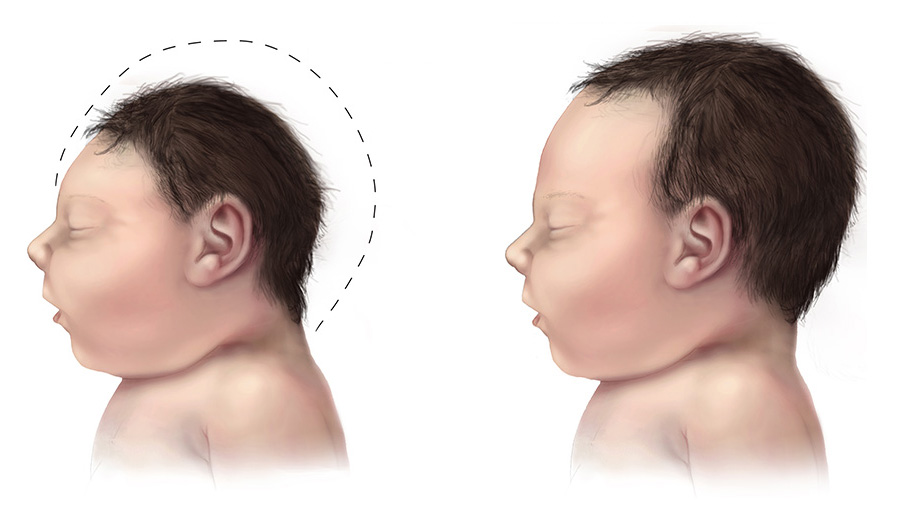
The researchers now knew that Zika preferentially infects early neuronal stem cells. But how did this lead to microcephaly? Once again, the social and collaborative aspects of research made all the difference.
Horvath researches metabolic cell functions and was working with a molecule known as TANK binding kinase 1 (TBK1), a crucial enzyme for innate immune signaling and cell proliferation. Under normal conditions, TBK1 is important for the proper functioning of the centrosome, a component of cells that drives cell division. The team discovered that following Zika infection, TBK1 was redirected to the mitochondria instead, robbing the centrosome of an essential enzyme and halting cell division. “Essentially what you are left with is a chimeric cell, with multiple nuclei and centrosomes but no cell division,” Sestan said. Although it is unknown exactly how or why TBK1 is necessary for mitosis, a crucial molecular process of Zika virus that gives rise to microcephaly had been uncovered for the very first time.
In addition, the researchers observed that other viruses known as TORCH (toxoplasma, other agents, rubella virus, cytomegalovirus, and herpes simplex virus) syndrome pathogens also caused brain defects in the developing brain. However, since numerous vaccines have been developed to prevent many TORCH pathogen infections, microcephaly was rarely observed until the recent outbreak of Zika in 2015. Since Zika may not be so unique, after all, in its ability to cause microcephaly, the natural question was to determine how we may be able to neutralize this virus, just as we have done with the other viruses of this class.
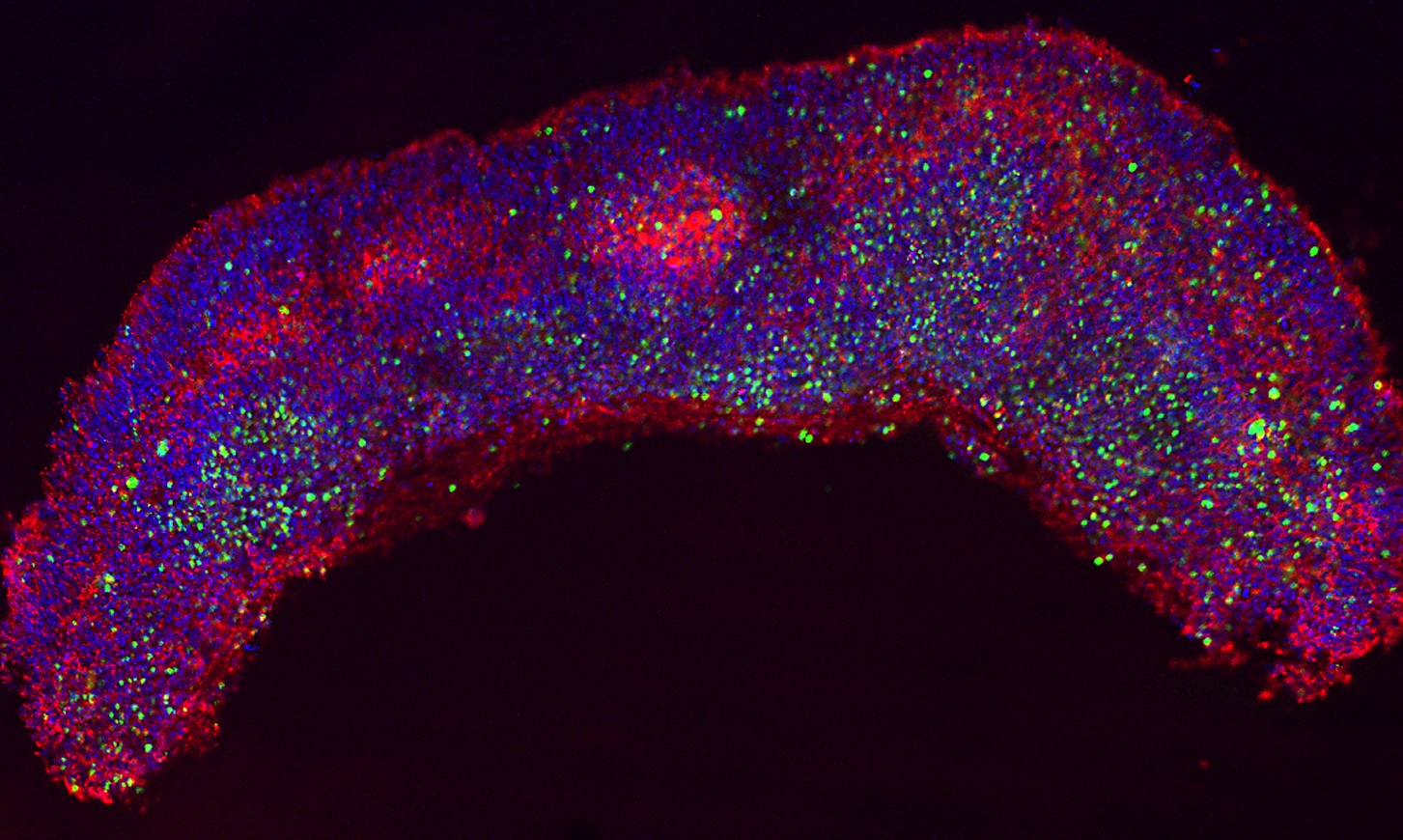
In the past, antiviral drugs, such as nucleoside chain terminators, have been effectively used against other flaviviruses, which includes hepatitis C and Zika virus. Now, the investigators have found that in cell cultures, the antiviral drug, Sofosbuvir, not only protects NES cells from Zika-induced cell death, but also inhibits the misdirection of TBK1. While the toxicity and pharmacology of these molecules are unknown, these findings present promising new leads for the future of Zika virus treatment.
One piece of the puzzle
Have researchers discovered the missing link between Zika and microcephaly? While the study is a major step in understanding Zika virus, many questions remain unanswered. It remains unknown why Zika virus preferentially infects NES cells and radial glial cells or how exactly the virus penetrates both the uterus and the developing brain, two highly protected areas under normal conditions. The molecular functions of TBK1 also require further investigation, and the researchers hope to derive mice models that lack this crucial enzyme. If TBK1 is necessary for mitosis in the developing brain, these mice should be microcephalic. Meanwhile, the absence of microcephaly in Zika cases in Colombia, as well as in related virus cases, such as Dengue fever, need to be addressed. “This paper is a rock solid brick in the wall of understanding,” Lindenbach said. “However, there are many more bricks that need to be put in place.”
Beyond discerning a piece of the Zika puzzle, the group’s research also illustrates the fruits of collaborative research. The researchers acknowledged that such an extensive, multidisciplinary study would have been impossible to accomplish through a single lab. Sestan observed that, as his lab works in brain development, they would never have looked into the virology methods ultimately used to study Zika. “That’s what I like about science. Every day is different, and that’s what motives me,” he said. It is that sense of optimism that drives important discoveries with the potential to bring health improvements to suffering regions around the world.
Further Reading: França, Giovanny V. A., Lavinia Schuler-Faccini, Wanderson K. Oliveira, Claudio M. P. Henriques, Eduardo H. Carmo, Vaneide D. Pedi, Marília L. Nunes, et al. “Congenital Zika Virus Syndrome in Brazil: A Case Series of the First 1501 Livebirths with Complete Investigation.” The Lancet 388, no. 10047 (2016): 891-97.
About the Author: Anson Wang is a senior in Davenport College majoring in Molecular Biophysics & Biochemistry. He currently works in the lab of Dr. Charles Greer studying the organization and morphological characteristics of axonal growth cones in the olfactory system. Outside of the sciences, he plays the clarinet for the Yale Concert Band and dances Bhangra on Yale Jashan Bhangra.
Acknowledgements: The author would like to thank Dr. Nenad Sestan, Dr. Brett Lindenbach, Dr. Marco Onorati, Dr. Andre M.M. Sousa, Fuchen Li and Zhen Li for participating in the interview and gathering the figures for this article, as well as for their enthusiastic research.
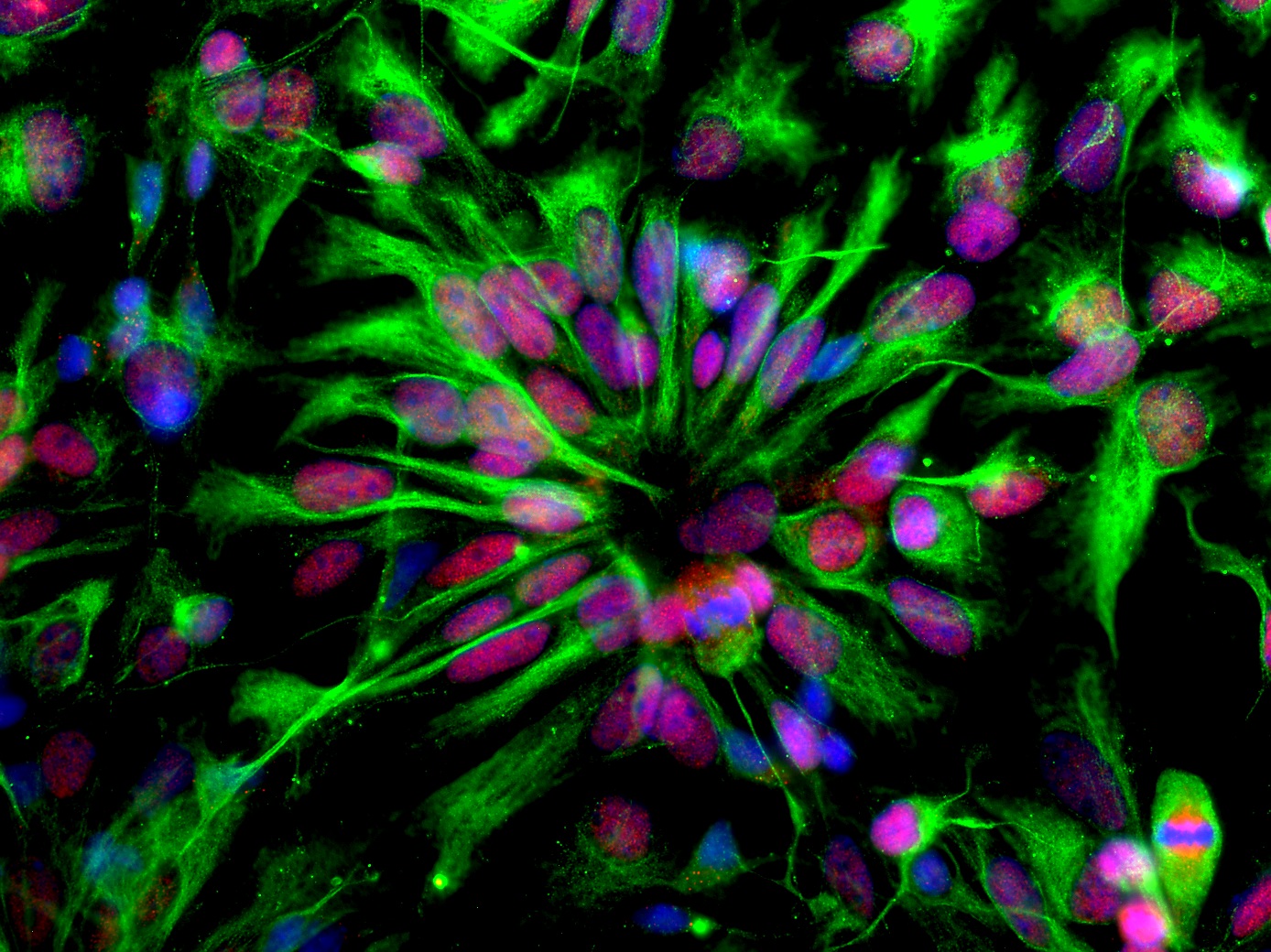
Cover image: Human neuroepithelial stem cells self-organize into rosette-like patterns, resembling the developing neural tube. They were used as an in vitro model to understand how Zika virus infection works and to block its proliferation through drug treatment. Image and caption courtesy of Dr. Marco Onorati
The original paper can be found here.