You’ve heard the news—bacteria have been quickly evolving immunity to the antibiotics that we’ve long used to fight them off, and diseases that are now easily treatable could come back in full force. We desperately need new antibiotics, and yet few pharmaceutical companies are willing to invest in developing a product that natural selection will so quickly render obsolete.
That’s why a team of Yale chemists, led by chemistry professor Seth Herzon, postdoctoral associate Stephen Murphy and graduate student Mingshuo Zeng, have come up with a new way to synthesize the antibiotic pleuromutilin, known to slow the process of bacterial resistance. It isn’t the antibiotic itself that was significant about the discovery—it’s been around for decades—but rather the process of creating it, which potentially will allow scientists to create more potent forms of the drug. By devising a new synthetic scheme for pleuromutilin, they have opened the doors for a new world of potential antibiotics that can provide us new weapons in the fight against resistance.

We’ve all grown up in an antibiotic world. Ever since Alexander Fleming’s accidental discovery of penicillin in 1927, lifespans have grown longer and many diseases across the developed world have been all but eradicated. But even as antibiotics changed the way that we live our lives, a new threat began to emerge. Rampant antibiotic use promotes the survival and proliferation of bacteria with special mutations in their genes that render antibiotics ineffective. Over time, more and more strains of antibiotic-resistant bacteria began to appear as only those with protective mutations survived rounds of antibiotic treatment.
Though we desperately need new antibiotics to counter the rising threat of resistance, that isn’t what’s happening, there are fewer and fewer new antibiotics being developed. “The reason that we have so much resistance and no new drugs is this really weird, twisted conflation of economics and evolution,” Seth Herzon said. Antibiotics aren’t like other drugs. Patients usually only use them for short periods of time, and existing ones are, in Herzon’s words, “dirt cheap.” And worst of all, antibiotics often last only a few years before resistance develops in bacteria, making the actual lifetime of an antibiotic much shorter than other drugs.
“The result is that there’s a terrible downward economic pressure not to do this,” Herzon said regarding antibiotic development. And while funding has increased slightly for small biotech startups in the past few years, far more new antibiotics are needed than are currently being made.
A longer-lasting antibiotic
In 1951, researchers at Columbia first isolated a powdery white substance from the edible mushroom Pleurotus mutilis and showed that it had antibacterial properties, naming it pleuromutilin. It was not until 2007, just as the antibiotic-resistant bacterial strain MRSA was making a global appearance in a major outbreak, that the very first antibiotic derived from pleuromutilin, retapamulin, was approved for human use. But retapamulin is different from other antibiotics; as of 2014, no bacterial resistance against the drug has emerged during the seven years that it’s been on the market. This is a much longer lifespan than the couple of years most antibiotics last before emerging resistance makes them obsolete.
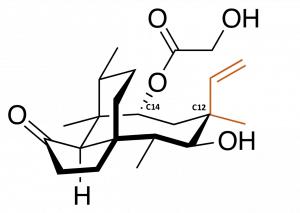
Modifying in the middle
Scientists have been exploiting this feature of pleuromutilin derivatives by developing a large number of variants through a process known as semisynthesis. Unlike a total synthesis, in which the product is made from scratch, semisynthesis involves starting with chemical compounds isolated from natural sources and then chemically modifying them. In this case, this has involved taking purified natural pleuromutilin and typically modifying it at single carbon atom, called C14 because of its position in the molecule. C14 is attached to a chemical group that is easily removed and replaced with other, customized groups that afford the chemical new functionality without resorting to complex synthetic methods. More than three thousand derivatives have been made by changing the chemical groups attached at only that position.
However, there are many more positions that could theoretically be modified. A study showed that changing the arrangement of chemical groups at a different carbon atom, C12, can afford the molecule some degree of efficacy against a group of harder-to-treat bacteria. These bacteria are distinguished by their outer membranes, and are harder to treat because this membrane blocks the uptake of many traditional antibiotics, including C14-modified pleuromutilins. While C12 modifications are theoretically possible by semisynthesis, they are much harder to access, and many other positions that could also potentially afford a wider range of effects cannot be accessed at all.
“As a chemist, there’s an obvious hole here,” Herzon said. “What we don’t have is a way to modify the other positions in the molecule.” What they needed was not pleuromutilin but a way to make pleuromutilin, and therefore a way to vastly broaden the substance’s antibiotic potential.
Starting from scratch
Expanding the number of possible pleuromutilin derivatives required the researchers to start from the very beginning. “What we’re doing is a total synthesis of pleuromutilin from scratch,” said Stephen Murphy, who spearheaded much of the technical work on the project. “That gives us total control of the compound, so we can explore a broader chemical space.”
The individual steps of the synthesis can be modified to yield a wide variety of different products. The full synthesis process involves a total of seventeen steps, transforming easily purchased chemicals into the final product, pleuromutilin. But synthesizing the product isn’t the real goal. “What we really care about is making pleuromutilin-like structures,” Herzon said. “With this synthesis in place, we’ve identified the viable pathways to these structures.” Now that the researchers have the essential blueprints to build this molecule, they can make modifications to any part of it at any stage of the building process.
They first reported their synthesis in June, and in the same report demonstrated that the final steps of the synthesis could be easily modified in order to create pleuromutilin derivatives with a flipped arrangement about C12. The total synthesis allows for many more options to make antibiotic derivatives that have never been explored. Because they can begin making modifications from earlier intermediates in the process rather than working backwards from the finished product, they can now access synthetic routes to pleuromutilin-like molecules modified in ways that would be impossible by semisynthesis, with functions that have never been studied. Members of the Herzon lab are well on their way to testing these new molecules for improved antibiotic properties.
“Right now we’re shooting around in the dark,” said Herzon, describing the search for the next effective pleuromutilin-based antibiotic. “Once we find something more active, we can drill down and further refine structure with more precision.”
The future of antibiotics
Pleuromutilins are a long-lasting family of antibiotics, but they aren’t eternal. It’s hard to predict the future of antibiotics, but many would claim that synthesizing more antibiotics now, even long-lasting ones, is only delaying the inevitable—total antibiotic drug resistance. Herzon agrees.
“We are basically just kicking the can down the road, but it’s the best option that we have right now,” he said. “We’re putting a patch on the antibiotic resistance crisis.”
For now, though, it’s quite a patch. By developing a synthetic scheme, they’ve not only achieved a breakthrough for synthetic chemistry, but they’ve also opened the doors to creating new weapons in the fight against antibiotic resistance.
About the author: Sam Berry is a junior in Ezra Stiles majoring in Molecular Biophysics & Biochemistry. He works in the in the lab of Alanna Schepartz, where he studies protein delivery by cell-permeant miniature proteins.
Acknowledgements: The author would like to thank Professor Herzon and Dr. Stephen Murphy for their time and enthusiasm when discussing their research.
Extra reading:
Murphy, Stephen K., Mingshuo Zeng, and Seth B. Herzon. “A Modular and Enantioselective Synthesis of the Pleuromutilin Antibiotics.” Science 356, no. 6341 (June 6, 2017).