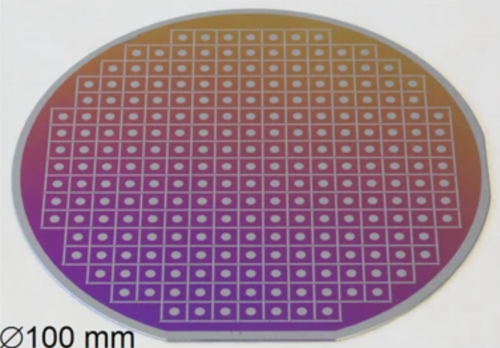
Image courtesy of Sebastian Kube, et al.
Despite their prevalence, the complex and diverse nature of metallic glasses remains poorly understood. Particularly, the amorphous and random atomic structures of metallic glasses clash with our conception of metals as orderly and crystalline—and for a good reason. Metals are ordinarily crystals, possessing an atomic spatial symmetry, and are thus much stabler and form easily. On the contrary, metallic glasses, being glasses, are inherently disordered and unstable—and, therefore, very difficult to create. In fact, for most metallic liquid mixtures, it often takes a cooling rate of trillions of kelvins per second to freeze the disordered atoms into a metastable metallic glass. However, a select few mixtures—narrowly defined compositions of some alloy systems—can be viably formed in bulk.
So, what determines these alloys’ glass-forming ability (GFA)—how easily they turn into glasses? Fragility, the relation between the temperature and viscosity of the alloy’s glass-forming liquid state, has long been thought to be a determinant of GFA. If the atoms have to trudge through a more viscous medium while cooling (lower fragility), it should take longer to array themselves, increasing GFA. Interestingly, it appears that this logic does not align with recent findings.
In a recent publication in Nature Communications, lead author Sebastian Kube (Yale SEAS PhD ’21), now a postdoc at UC Santa Barbara, and Jan Schroers, Yale Professor of Mechanical Engineering & Materials Science, helped develop a pioneering technique, the film inflation method (FIM), to study this relationship. Using sputtering “plasma” guns, they deposited magnesium, copper, and yttrium atom-by-atom onto a silicon substrate divided into wafers. With these synthesis conditions, the magnesium-copper-yttrium alloys formed glasses over a much wider composition range—over which the viscosity can then be probed. Moreover, this technique synthesized a gradient with large numbers of composition ratios in parallel, a task that would have taken lifetimes with previous methods. With this technique, they applied a constant gas pressure to the freestanding metallic films, inflating them into a bubble. Using lasers, they delicately measured the bubble’s height over time and, in turn, were able to infer information about the respective compositions’ fragilities.
To the shock of Kube, Schroers, and their colleagues, fragility was hardly a determining factor of GFA. “I still believe in it. I believe that… a [lower fragility] liquid is the better glass former,” Kube said. Indeed, the stark contradiction with the assumed importance of GFA, a mainstay of the metallurgy community, underlines just how nebulous the nature of metallic glasses remains to materials researchers. Going forward, Kube hopes to re-explore this problem in greater detail. “My long-term plan is to build a better version of this [experiment]… for ten or so alloy systems,” remarked Kube, “and we will get much more information… about how fragility affects GFA and interacts with other properties.” Indeed, Kube is eager to elucidate this mystery of glass-forming ability and fragility, an undertaking with major implications for additive manufacturing and materials engineering—of course, with only more twists and turns to be expected along the way.