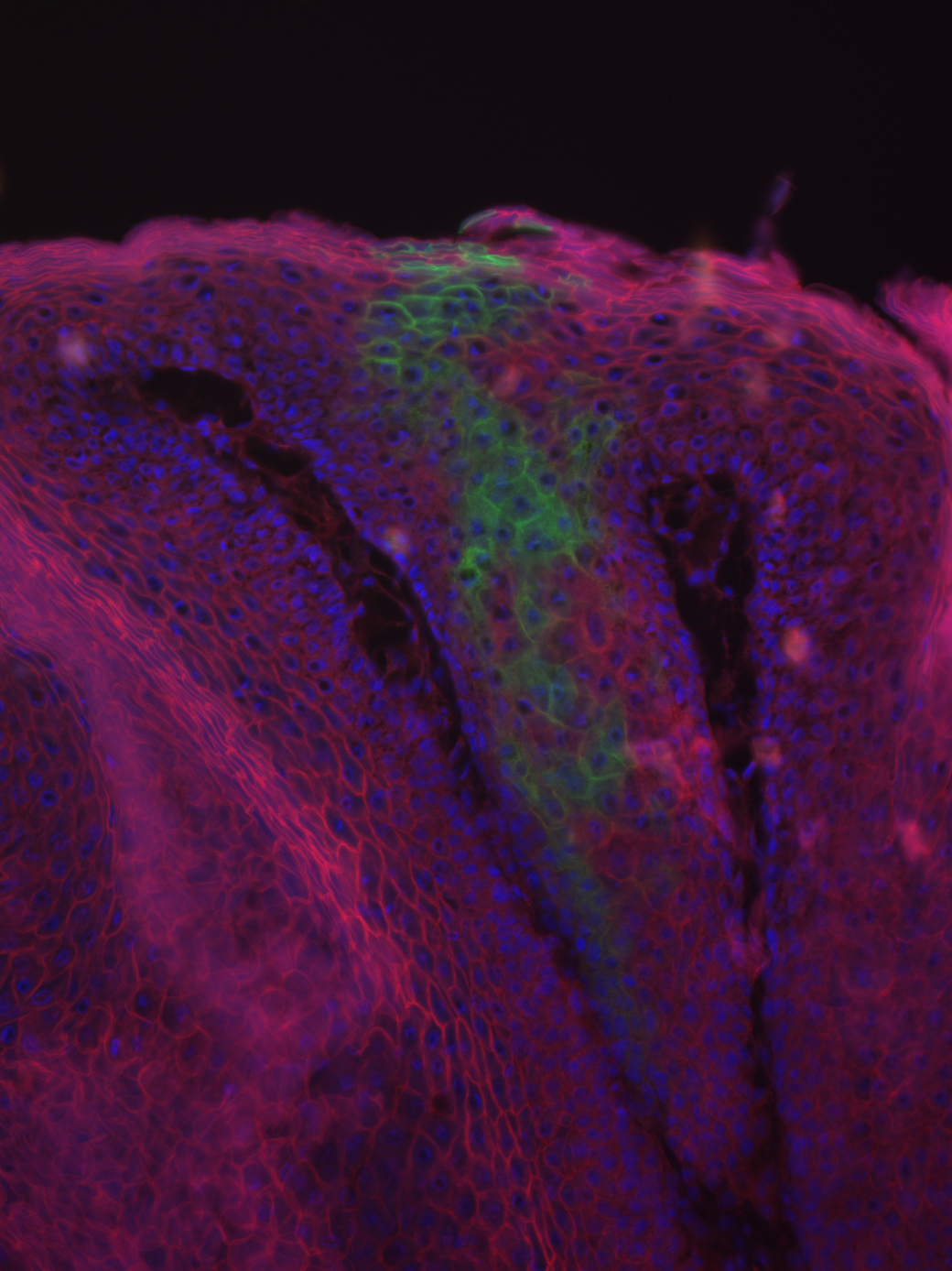
After decades of focused research, the molecular origins of cancer are becoming increasingly well understood. Cancerous tumors result from the uncontrolled growth of cells due to multiple mutations and/or external factors, the start of which is known as carcinogenesis. In order to tackle cancer, we not only need to understand what effects each contributing player has but also how the effects are carried out. Transcription factors, molecules in cells that regulate how much of a specific gene is expressed, are one of the common targets of investigation. In particular, one transcription factor known as Nfatc1 has been implicated in hair growth and skin carcinogenesis, though the nature of the relationship is poorly understood. A team of researchers led by associate professor Valerie Horsley has recently shed light on this issue in their paper in Molecular Biology of the Cell, showing that loss of Nfatc1 expression significantly lowers the rate of chemically-induced carcinogenesis in the skin.
To understand the initiation of skin cancer, the Yale researchers began by focusing on a cell type known to give rise to cancer cells. These cells, known as hair follicle bulge cells, are a cluster of stem cells situated in hair follicles for hair growth. Previous research has found that Nfatc1 is highly expressed in bulge cells, and molecular signals related to Nfatc1 are especially active. Aside from its role in hair follicles, Nfatc1 is also responsible for activating T cells in immune responses. To suppress these immune responses during organ transplants, the drug cyclosporin A is used to inhibit calcineurin, an activator of Nfatc1. Previous reports of enhanced hair growth and squamous cell carcinoma under cyclosporin A treatment, as well as contradictory studies showing that Nfatc1 overexpression promotes skin tumors, triggered the interest of the Yale researchers to investigate the effects of perturbations in this pathway.
In this paper, the Horsley lab studied the significance of the calcineurin-Nfatc1 pathway in skin cancer using DMBA and TPA, standard chemicals for cancer cell initiation and proliferation in mice cancer studies. Using a series of molecular biology experiments, they found that Nfatc1 is positively related to the rate of tumor formation by affecting the expression of DMBA-metabolizing enzymes. Apart from the main conclusions drawn, these findings also accentuated the heterogeneity of carcinogenesis: skin tumor cells could be generated from a variety of cell types, and different cell types might also respond differently to mutagens. These implications are significant for both clinical analyses and cancer research. Future studies using genetic instead of chemical carcinogenesis models, and experiments to unveil other substrates of calcineurin, could reveal more of the roles of calcineurin and Nfatc1 in cancer.
When talking about the research process behind the paper, Horsley reflected upon the unexpected nature of scientific experiments. “That’s the kind of the excitement of science —you go places where you’d never dreamed you would,” said Horsley. She also emphasized the remarkable contribution of undergraduate researchers in this investigation, which is encouraging for undergraduates interested in pursuing research at Yale. Horsley looks forward to new experiments to further unveil the processes behind skin carcinogenesis.
List of sources:
[1] Goldstein, J. et al. Loss of endogenous Nfatc1 reduces the rate of DMBA/TPA-induced skin tumorigenesis. Mol. Biol. Cell. mbc.E15-05-0282 (2015).
[2] Horsley, V. Personal communication.
Featured image courtesy of Professor Valerie Horsley. It is a microscope image of tumor showing cells derived from the bulge (green).