Batteries are found in everything from cell phones to cars, quietly powering our everyday existence. With rising pressures to find more renewable sources of energy, batteries hold immense potential to do even more — for example, to store the excess energy from solar cells and wind turbines to release when demand is high, or to fuel efficient and easily-rechargeable electric vehicles. However, news reports and recalls on consumer products have revealed the potential danger beneath these batteries’ noiseless exteriors: overheating laptops, vehicles catching fire, and hoverboards exploding underneath people’s feet. The primary culprit for these hazards is a buildup of heat in lithium ion batteries, which are high in energy density, inherently reactive, and easily short-circuited. If a battery’s temperature exceeds approximately 150 degrees Celsius, it can catch fire and cause an explosion.
Earlier in January, Zheng Chen, Yi Cui, and Zhenan Bao of Stanford published their work on a new technology to solve this problem in lithium-ion batteries. Using a polymer material embedded with nickel nanoparticles with spiky surface features, they invented a self-regulating film that can shut down batteries in case of overheating and short-circuiting. “Our inspiration [was] to solve the general safety issues related to batteries,” said Chen, the lead author of the paper. “It could be small scale or large scale batteries in different formats; all are subject to safety issues.”
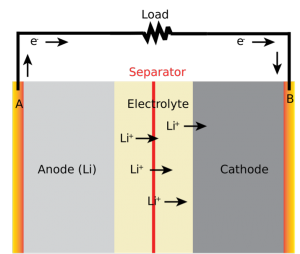
To understand the mechanism for these self-regulating batteries, a basic understanding of the hazards of traditional lithium ion batteries is necessary. Why exactly do lithium ion batteries catch fire? Despite their reputation, standard lithium batteries are, for the most part, reliable. They are commonly used due to their high energy, power density, and reliability, but they can also be dangerous if the batteries become damaged. In a functioning battery, lithium ions flow in a balanced circuit from an oxide cathode, to an electrolyte solution of lithium salts and organic solvents, then to a carbon anode. Damage to the thin barriers separating the cathode and anode, however, can create an internal short-circuit. When short-circuited, overcharged, or otherwise misused, the batteries can reach dangerously high temperatures, leading to “thermal runaway” — a series of chemical reactions that raise internal temperature and pressure until the battery bursts into flames.
To combat this issue, the researchers devised a system to decrease the conductivity of electrodes at high temperatures. In a previous project, Professor Bao had created a device that monitored body temperature, so the team fabricated a similar material for batteries.
They encountered new difficulties, however, since the battery film quickly degraded when exposed to the chemicals inside batteries. To prevent degradation, the team coated the nickel particles with conductive graphene, a thin layer of carbon atoms. “The nickel provides the composite with electrical conductivity, the graphene coating layer on the nickel surface provides them electrochemical stability, and the polyethylene is the matrix to hold such particles and can expand and shrink depending on increasing or decreasing temperature,” Chen said.
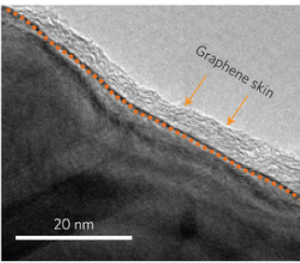
When attached to battery electrodes, the particles in the film conduct electricity. But when the battery heats up above a certain temperature, thermal expansion causes the plastic to stretch. As a result, the particles in the film spread apart, halting electric current and shutting off the battery. This process occurs remarkably quickly, with conductivity dropping by a factor of 107-108 in a mere second. After the film cools, resistance decreases and the film relaxes, allowing electron flow to continue. Consequently, the thermal switching of the batteries is quick and reversible.
This is not the first project that attempted to eradicate the dangers of overheating batteries. In an earlier design, Cui created a lithium-ion battery with an “early-warning system” to detect abnormal operating conditions. Cui and his colleagues decided to build a “smart separator” of copper between the anode and cathode of the battery. By sensing the voltage difference between the anode and cathode, the copper could recognize abnormal conditions to determine when the battery should be removed to prevent short-circuiting. Elsewhere, at the University of Rhode Island, Ronald Dunn has experimented with including flame-retardants in lithium-ion batteries.
So how is this new design different? In previous designs of safe batteries, the mechanisms for shutting off overheating batteries were irreversible; the batteries could not be used after overheating. The reversibility of thermal switching is truly innovative. When the researchers repeatedly heated their battery with a hot-air gun, the film was very resistant to high temperatures and still reliably conductive after twenty cycles of being switched on and off.
Can this polyethylene film eventually be implemented on a larger scale? Chen thinks so. “Both the components and fabrication process are low cost, so we don’t think there will be a problem for scaling up,” Chen explained. Until then, he and the other researchers hope to continue with their research to improve the batteries further, decreasing the overall thickness of the composite film and increasing its conductivity at room temperature. “We still need to improve our materials design and processing,” Chen said.
If a self-regulating, temperature selective material was used in batteries, it could potentially maintain good battery performance at normal temperatures, but more importantly, it could provide a reusable safety mechanism to shut down at high temperatures. This new technology may decrease the risks associated with our smartphones, laptops, and electric cars. And perhaps even hoverboards may return to the Yale campus.