POW! You fall to the ground. Bystanders call 911. EMTs transport you to the hospital. Your breaths are irregular, your pulse is slow, yet your heart pumps as much blood as it can with every beat. Your symptoms meet the telltale signs of increased intracranial pressure—a buildup of pressure inside the skull—from traumatic brain injury (TBI). Your doctors decide to perform neurosurgery to alleviate the pressure. However, neurosurgery can lead to swelling that also increases your intracranial pressure, throwing the brain’s continuous checks and balances off rhythm and leading to potential brain damage. Following surgery, how can doctors make sure your brain sticks to the beat?
“Intracranial pressure monitoring is the mainstay of how we manage some of these [TBI] patients early on,” said Wilson Ray, assistant professor of neurological and orthopedic surgery at the Washington University School of Medicine in St. Louis. Ray and John Rogers, professor of materials science and engineering at the University of Illinois at Urbana-Champaign, teamed up to develop bioresorbable devices to monitor brain conditions like intracranial pressure.
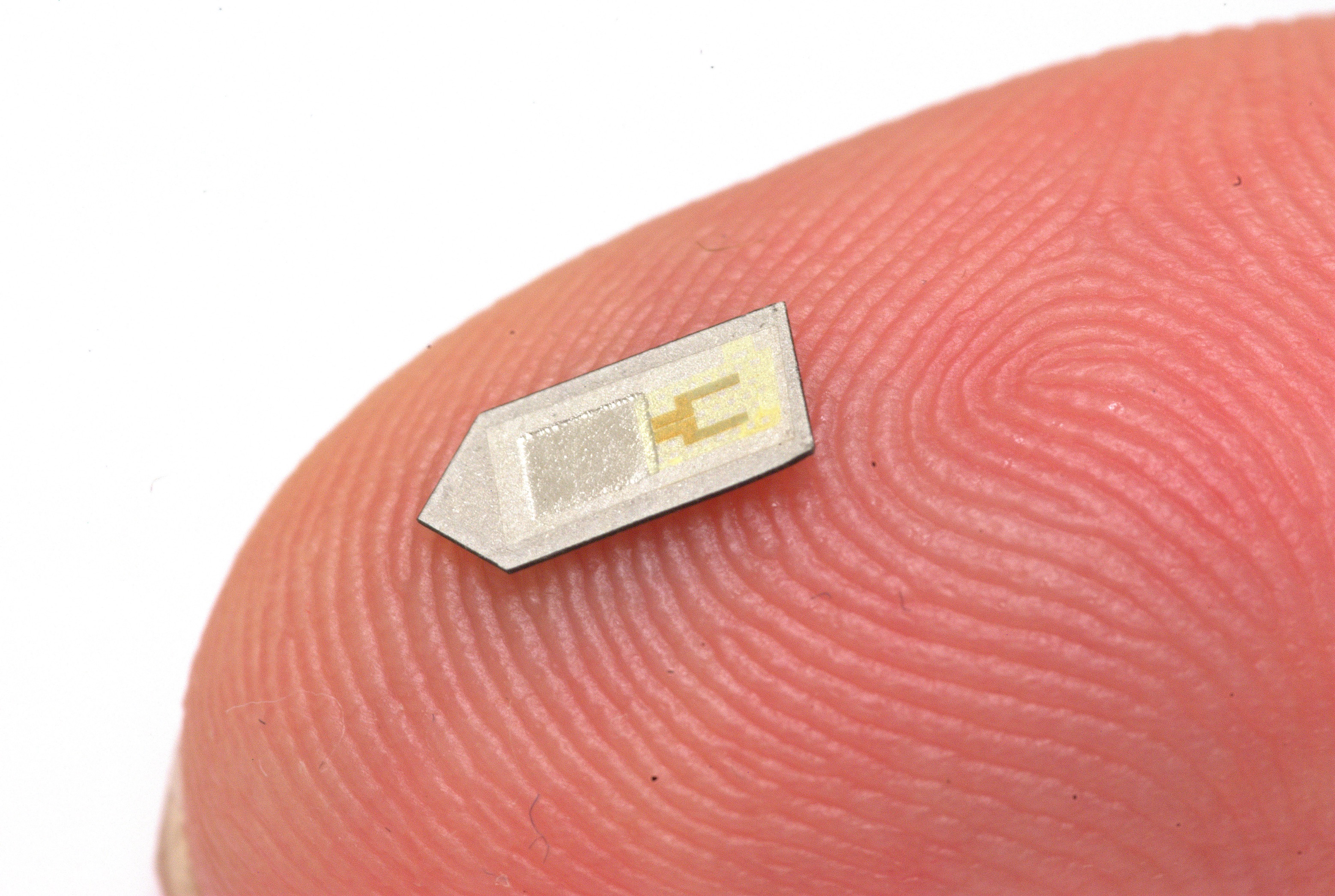
“The devices themselves are made out of several types of materials, configured in a multi-layer stack to provide the kind of functionality we need,” Rogers said. The devices have two main components: a sensor and an electrode system. The sensor is placed within the brain, resting on a pen-tip-sized base. Wires connect it to the electrode system—a dime-sized electric circuit placed between the skin and the skull—that stores information in a near-field communication (NFC) chip. Excluding the chip, all these materials are biodegradable, breaking down through natural processes like banana peels. The device uses the biodegradable chemical PLGA as a protective coating to lengthen its lifetime. Placed underneath the sensor to form an air cavity, a membrane made of PLGA also bends in response to environmental changes to help the sensor measure conditions accurately. After the materials biodegrade, bodily fluids like blood can absorb and remove the byproducts from the body, making the device bioresorbable.

Depending on the type of sensor, the device can measure pressure, temperature, or flow rates among other conditions specific to the sensor’s location. The sensor converts the condition information into electricity that flows through the wires for the electrode system to process. The NFC chip exchanges information on internal conditions only when an external reader hovers within 25 millimeters of the chip’s location underneath skin. The same technology powering Apple Pay and Google Wallet may soon advance health monitoring.
However, the devices have not entered hospitals yet. Ray and Rogers conducted the first round of device testing in artificial cerebral spinal fluid (CSF) and rats. CSF is the clear, colorless, shock-absorbing liquid that helps our brains stay afloat inside our skulls and flushes away the waste produced by our brains throughout the day. Observing that the device dissolved completely in artificial CSF, the researchers predicted that the body would naturally wash out its byproducts. “The body tries its best to remove foreign bodies from [itself],” said Rory Murphy, a chief resident in neurosurgery at the Washington University School of Medicine in St. Louis. The researchers studied test implants in rats to ensure that the dissolved byproducts were treated as welcomed guests rather than foreign invaders. Otherwise, an immune response would have signaled the body’s security forces to attack. Fortunately, rat brains were very hospitable.
Current monitoring implants do not receive the same welcome. They do not biodegrade, they act as platforms for infection while they remain in the body, and they outlast their brief period of clinical use. “That first 24, 48, and certainly 72 hours is where you are making some of those critical decisions regarding on-going medical management versus surgical intervention,” Ray said. Although current implants do provide accurate condition information following TBI or surgery, doctors must perform additional surgery to remove them. While preventing a potential immune response, this creates yet another opening for health complications.
In rats, the new bioresorbable devices matched the accuracy of current monitoring implants without the associated side effects. Nevertheless, Murphy stresses further testing must be done to guarantee that the devices are completely safe for use in humans. If proven safe, researchers could convert the monitoring device into a medication. “We have approaches to do that,” Rogers said. He believes the device could be modified to electrically stimulate a specific brain area, allowing clinicians to provide electrotherapy remotely. Likewise, the electrodes could be programmed to release prepackaged drugs. “There is going to be tremendous opportunity as to which direction [device applications] will go,”
Ray said.