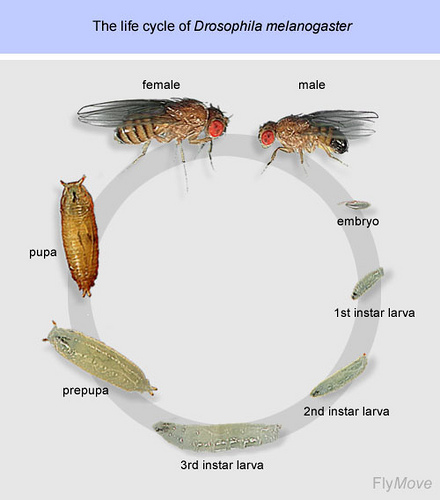
Fruit flies may be annoying pests, but a team of biologists at the Howard Hughes Medical Institute believe that they could serve a higher purpose than target practice for a fly swatter. Despite their diminutive appearance, fruit flies—also known as Drosophila melanogaster—could hold the key to understanding and treating cancer growth in humans. The team’s recent study probes the mechanisms for how hormones are regulated in growing juvenile flies; their efforts have shed light on how interactions of different growth hormones can affect the final size of the flies. The research team believe that the interactions they elucidated are similar in nature to those implicated in tumor growth in humans, establishing the possibility for further studies that could assist in the development of new cancer therapies.
One molecule known to be critical to regulating growth in insects is juvenile hormone (JH); however, a comprehensive understanding of the mechanism by which it controls size has not been established. At the onset of the research effort, the team knew that JH is implicated in the metamorphosis of insect larvae. In order for the process to begin, the larvae must reach a hormonally regulated critical weight. Modulating this critical weight affects the size of the insect larvae when they begin metamorphosis. As such, there also are changes in the adult size of the insects. Though JH is known to be involved in controlling the timing of metamorphosis, its role remains unclear. Previous studies have shown that it is not directly linked to critical weight; if insects are fed a diet containing JH mimics, the timing of metamorphosis is unaffected.
In their paper published in the Proceedings of the National Academy of Sciences, the researchers, led by Christen Mirth, explored this phenomenon with a novel approach. The team removed the insects’ corpora allata (CA), the organ that produces JH in Drosophila melanogaster, and observed the effects on the growth rate and timing of metamorphosis. The researchers discovered that larvae with a removed CA—thus lacking JH—matured at smaller sizes than wild type flies. The timing of metamorphosis, however, was unchanged, suggesting that the lack of JH primarily caused a change in the growth rate.
The researchers also explored the effects of the absence of JH on other related growth hormones. The team discovered that they could reverse the observable reductions in growth rate by artificially changing the levels of two other growth-related hormones, ecdysone and insulin. According to Mirth, this suggests that “crosstalk” between JH and these other hormones helps to control both growth rate and growth time.
“JH acts to fine-tune growth rates,” Mirth said in an interview for Science Magazine. This means that JH elicits changes in size by coordinating with ecdysone and insulin, rather than acting independently, as was previously assumed.
These findings have implications that reach much further than fruit flies themselves. Much akin to the interactions at play in insect growth hormones, the researchers hypothesize that there is significant crosstalk between androgens, estrogens, and insulin signaling in human tumor growth. Thus, Mirth and the rest of her team hope that subsequent studies can benefit from a more nuanced understanding of growth hormone interactions, in order to better probe the mechanisms of the runaway cell division that characterizes cancer.

