Imagine you could shrink yourself to the size of a neuron and explore the pathways inside the human brain. You could map the networks between neurons and listen to the secret molecular conversations occurring constantly at the connections between them. Based on what the neurons are saying, you could gather inside information about whether or not the brain is healthy. Unfortunately, from an outside perspective, these conversations are hard to make out.
The human brain is incredibly complex and mysterious—it contains about 100 billion neurons that encode all our thoughts and movements. These billions of neurons are wired together by an estimated 100 trillion synaptic connections. At synapses, neurons talk to each other, using chemical messengers called neurotransmitters to send a variety of signals and information. Now for the first time, we can eavesdrop on live neurons, thanks to a technique developed by professor Richard Carson and postdoctoral fellow Sjoerd Finnema at the Yale PET center.
Imaging synaptic density can provide a wealth of information on the processes occurring inside the brain. Synapse loss has been linked to a number of neurocognitive disorders including Alzheimer’s, Parkinson’s, autism, depression, and schizophrenia. In the past, the only way to image synaptic density was by examining brain tissue during an autopsy. Now, Carson, Finnema, and their team of researchers have developed a novel method of synaptic imaging that can be used in living patients. This minimally invasive method has huge potential in diagnosing and monitoring the progress of neurocognitive disorders.

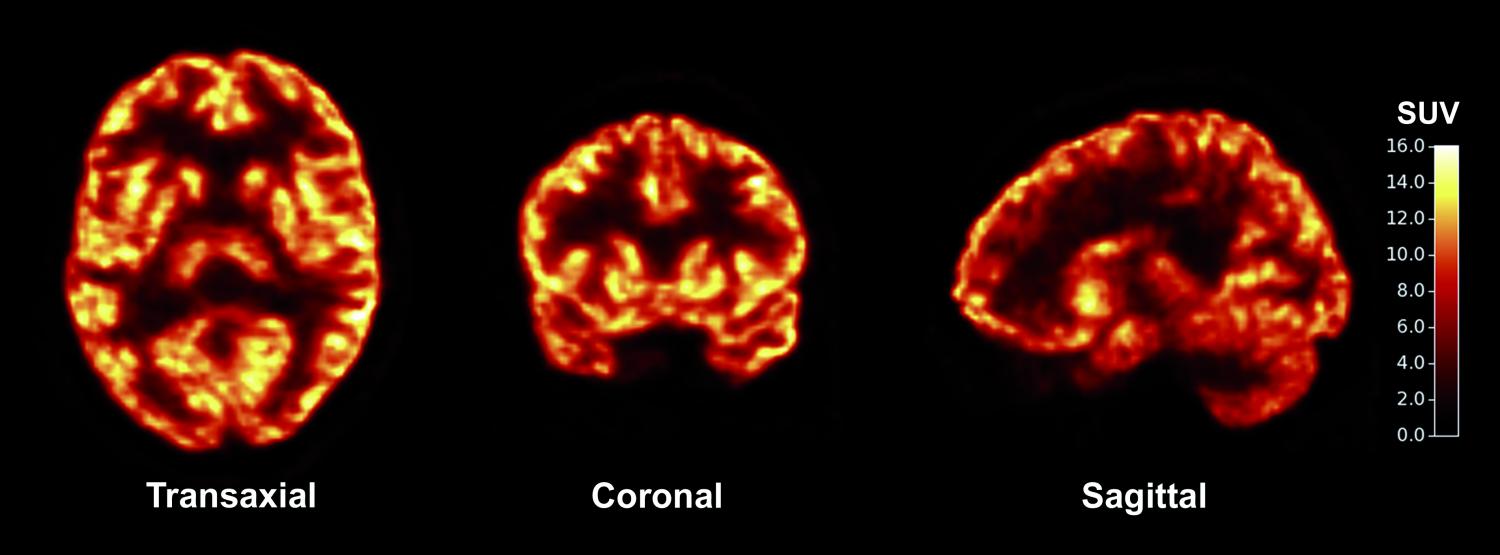
The new method developed by Carson and Finnema uses positron emission topography (PET), a technique commonly used by neuroscientists, to observe the activity of the living brain. During a PET scan, a radioactive tracer such as a glucose-like molecule is injected into the patient, and the areas of localization of the tracer reveal where the brain is active. The PET Center team developed a new tracer called [11C]UCB-J, which binds to a protein found in synapses and thus reveals where neurons are talking.
A new blade
PET imaging is a flexible technique with a number of clinical applications. It can be used to monitor the activity of the body and detect abnormalities such as cancer, heart disease, and neurocognitive dysfunction. Depending on the type of radioactive tracer used, PET can target a wide range of tissues in the body, making it a multipurpose tool.
“PET is like a Swiss Army knife,” Carson said. “With one technique, you can perform many different functions, depending on which radioactive material you inject. Now we’ve added a new blade to our knife, using a new molecule to perform a different task.” Carson explained that with each blade added to the knife—with each new molecule developed—the Swiss Army knife becomes a more powerful device. With improved imaging techniques, scientists and clinicians will be able to better understand not just the normal function of the brain, but also what goes wrong in a diseased state.
“If you find the right molecule, you can image anything—so our goal was to develop a novel radio-pharmaceutical that can target a specific process in the human body,” Carson said. The new molecule that the researchers discovered, [11C]UCB-J, targets a protein called SV2A found specifically in the synapses of the brain. Neurons communicate with each other at synapses through the action of chemical neurotransmitters. One neuron releases a vesicle containing neurotransmitters into the synaptic cleft, and these neurotransmitters are picked up by its neighbor. SV2A is a protein that is embedded in presynaptic vesicle membranes. Because SV2A shows up consistently in all synapses of the living brain, PET imaging and quantification of SV2A can be used to determine synaptic density.

Testing the technique
To make sure that their new technique presented an accurate measure of synaptic density, the researchers performed a number of studies involving both animal and human subjects. They first injected [11C]UCB-J into olive baboons (Papio anubis). Within a few minutes of injection, radioactivity rose within the brain, with the signal highest in synapse-rich gray matter and lowest in synapse-poor white matter.
The researchers wanted to confirm that their new technique yielded similar results to previous postmortem techniques. They dissected the brains of the baboons and determined the levels of both SV2A and another synaptic protein that is considered the gold standard for measuring synaptic density. As expected, the levels of the two proteins correlated well. Furthermore, SV2A levels were similar whether determined by PET imaging or postmortem analysis, suggesting that PET is an accurate measure of where a protein is localized.
Five healthy human subjects were recruited to test the PET imaging technique. As with the trials with baboons, radioactivity rose in the brains of the human subjects within a few minutes of injection. Once again, the signal was highest in gray matter and lowest in white matter.
Repeating the procedure in three epilepsy patients, the researchers found that these patients displayed an asymmetric uptake of the radioligand with a loss of signal in the hippocampus and amygdala, two brain structures where the patients had suffered damage due to their epilepsy. This exciting finding confirmed that the new technique was sensitive enough to pick up differences in synaptic density between healthy patients and patients with a neurocognitive disease.
The future of synaptic imaging
In its healthy state, the brain is constantly re-wiring itself, trimming out some synapses and strengthening others. Synaptic pruning, for instance, is a process occurring throughout and after adolescence wherein neurons shed old connections to reinforce the efficiency of essential ones. When the brain makes mistakes in regulating its synaptic connections, disease can result. Synapses are destroyed in a number of neurocognitive diseases, and scientists are still seeking to fully comprehend the connection between synapse loss and disease.

Carson emphasized the importance of the new synaptic imaging technique for improving our understanding of neurocognitive disorders. “Alzheimer’s, Parkinson’s, schizophrenia, Huntington’s, autism, depression, traumatic brain injury, alcohol abuse…these are just some of the disorders where synaptic loss has been implicated,” Carson said. “We want to combine information from many imaging techniques to understand disease and disease progression.”
One of the researchers’ biggest targets is Alzheimer’s, Carson stated. The pathology of Alzheimer’s has been fairly well characterized: Alzheimer’s patients have abnormal structures called amyloid plaques and neurofibrillary tangles in their brains, and PET imaging techniques have been developed that target these specific structures. As the loss of synapses is also tied to loss of function in Alzheimer’s patients, Carson believes that synaptic imaging will add a new technique to the diagnostic toolbox, a new blade to the Swiss Army knife. “The addition of our tracer will help improve diagnostics. We would like to use multiple tracers to follow the progression of the disease over time, and this will be very valuable in both diagnosing and treating patients.”
However, more research is needed to validate the consistency and accuracy of the technique before it can be used widely. Carson envisions a greater number of clinical trials on larger patient populations in the near future. He hopes that the technique will soon be used not just by research scientists, but also by physicians, neurosurgeons, and pharmaceutical researchers. In the meantime, he is optimistic about collaboration to further develop the technique and hone this new addition to the Swiss Army knife of neurological diagnosis. “Many other PET centers around the world are working on this,” Carson said. “We look forward to working with them.”
Further Reading: Van Bommel, B., and M. Mikaylova. “Talking to the Neighbours: The Molecular and Physiological Mechanisms of Clustered Synaptic Plasticity.” Neuroscience & Biobehavioral Reviews 71 (2016): 352-61.
About the Author: Christine Xu is a junior in Saybrook College majoring in Molecular, Cellular, and Developmental Biology. She pursues research on treating neurocognitive disorders and spent the summer at Stanford working on a stem cell differentiation project. She also currently works in a neurodevelopment lab at Yale studying axon growth in the olfactory system. Besides writing for Yale Scientific Magazine, she enjoys playing classical piano and singing in her a cappella group, Pitches and Tones.
Acknowledgements: I would like to thank Dr. Carson for his time and his enthusiasm in sharing his work.
Cover image: The new synaptic imaging technique provides a picture of synaptic density in the living brain. Image courtesy of Science Translational Medicine