Scientists are stopping seizures at their source with a new, dry drug delivery method that is efficient, effective, and electrophoretic. This new strategy could swiftly and efficiently stop epileptic seizures. According to the CDC, epilepsy affects approximately 1.2% of the United States’ population, with 3 million adults and 470,000 children affected by this disorder. With such a high prevalence, finding an effective drug treatment that allows for precise control and manipulation of electrical neurochemistry with minimal side effects is paramount.
Epileptic seizures are characterized by abnormal neural electrical activity. While they can stem from many different causes, they are all inhibited by the neurotransmitter γ-aminobutyric acid (GABA), and many seizure treatments are geared toward facilitating GABA delivery, production, or reuptake inhibition. The two broadest categories of seizures are general and partial seizures, and the latter responds especially poorly to traditional systemic drug treatment, which affect the entire body.
Systemic drug treatments are not well-designed for treating many neural diseases and disorders in general due to the brain’s protective barriers, such as the blood brain barrier, and the drug’s inability to target specific neural sites. The blood-brain barrier prevents harmful chemicals in the surrounding fluid from entering the brain, and in order to penetrate this barrier, such a high concentration of drug would need to be present in the blood stream that it would have detrimental effects to other tissues in the body.
Many systemic psychotropic, or neurochemistry-altering, drugs that in lab experiments have indicated strong anti-seizure and remediating effects have also ultimately proven to have acute severe side effects, which causes these otherwise effective drugs to fail during clinical trials. Further, systemic treatments do not allow for a high level of precision and spatiotemporal control. This precision is critical if a drug is to address neural disorders, including focal seizures, which have a defined focal point or source. While there are other drug delivery methods, such as fluid delivery systems, that are directly implanted into the brain and, therefore, don’t have to contend with the blood-brain barrier, these mechanisms release the molecules of the drug in fluid. This fluid build-up can lead to increased pressure, local edemas, and alterations to nearby neural networks.
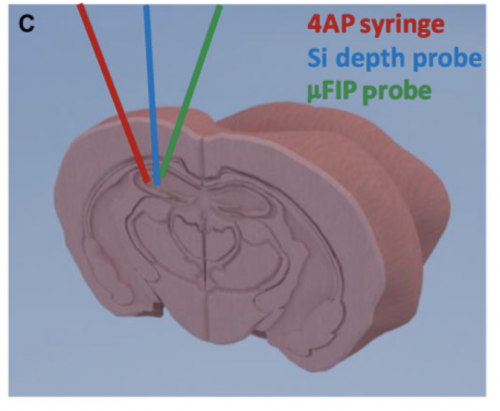
To address and circumvent these challenges to treating neural disorders, a team of researchers affiliated with the Aix Marseille Université and the University of Cambridge designed a microfluidic ion pump implant to deliver precise drug dosages more efficiently. This method of drug delivery features dry drug delivery capabilities, as it delivers the drug molecules without any solvent. Scientists successfully used this ion pump implant to detect seizures and immediately deliver precise amounts of positively charged ions of neurotransmitter that effectively ended the seizure-like event in mouse models. “These findings demonstrated the effective use of an implantable drug-delivery system in vivo [or in a living animal] to control seizures,” said Dr. Adam Williamson of the Aix Marseille Université, part of the team who developed this new technology. This technology integrates the principles of physics and biochemistry to create, in essence, an electrochemical circuit that can be implanted in the brain.
The microfluidic pump is strongly based on previous designs of ion pumps and functions as an electrochemical circuit. The implant is placed locally by the focal point of the seizure and the mechanism consists of a microfluidic channel, a gold source electrode, and two peripheral, plastic-coated electrodes. The two outer electrodes function to record neural activity. When seizure-like abnormal electrical neural activity is detected, a voltage is applied between an external electrode and the source electrode, which triggers the delivery of positively charged particles through a negatively charged membrane, the anion exchange membrane, to the target outlet that release the drug. Opposites attract as the positive drug treatment particles stored in the pump move across a negative membrane to the outlet site.
The microfluidic ion pump has the added advantage of being more sensitive to voltage and being incredibly efficient, transporting nearly all molecules to their intended target. In their study, the research team used a GABA solution known to inhibit seizure-like activity as the drug treatment. GABA induces the uptake of negative chloride ions, which decrease a neuron’s ability to fire a signal and, in turn, inhibit neural activity. Further, GABA is quickly broken down, which decreases the potential side effects of a high concentration of GABA.
Upon inducing seizure-like events in mice, the probe successfully recorded the abnormal activity and released enough GABA to readjust and regulate neural activity, thereby stopping the seizure at its source within seconds. After the probe had been implanted, no further seizures could be induced, illustrating this method is highly effective. Further, the GABA that was delivered to the seizure source site was quickly broken down, indicating that since the drug was so quickly degraded, it had little to no adverse side effects.
While this technology is very promising, as it obviates many formerly daunting challenges in treating neural disorders, this new technology is still limited in its applications. Most importantly, the treatment is reactionary. Since the mechanism senses and responds to abnormal neural activity, the treatment is only administered after a potentially dangerous seizure has started to occur. Even so, researchers have postulated that integrating algorithms that can potentially predict seizure onset with the microfluidic ion pump probe could prevent a seizure before it occurs. Further, treatments were administered to mice in an anesthetized state and the focal point of the seizure was already known.
The clinical implications of this new technology for an electrophoretic dry drug delivery method are broad. “A very optimistic person would say that we are close to developing an implantable device for the treatment of epilepsy, equivalent to Deep Brain Stimulation used in the treatment of Parkinson’s disease. I would encourage cautious optimism. Challenges will need to be individually investigated and integrated step-wise logically to move the technology forward,” Dr. Williamson said. While the new technology must be systematically refined, it has the potential to treat not just seizure disorders, but other deleterious chronic neural disorders like Parkinson’s. By minimizing adverse effects and extra solvent molecules and maximizing precision and efficiency of treatment in their electrophoretic dry drug delivery method, Dr. Williamson and his colleagues have developed has a wide range of future potential applications for treating neural disorders.