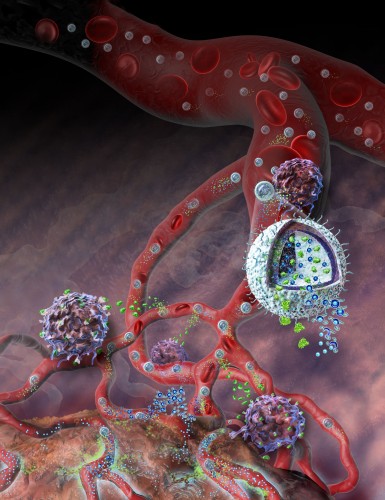
It reads like a scene straight from a 60s sci-fi movie. Tiny pods flood the bloodstream, each one a marvel of physical engineering and in hot pursuit of concealed enemy cells deep undercover. Upon detecting the offenders, the capsules detonate into a cloud of chemicals, sounding the body’s alarm. Within moments, the reinforcements have arrived: the full wrath of the body’s fleet of immune cells. With swift vengeance, the various lymphocytes make quick work of the exposed enemy cells, saving the day once again.
Although the reality is slightly less dramatic, within the walls of Yale’s Malone Engineering Center, what was once science fiction has now entered the realm of possibility. Associate Professor of Biomedical Engineering Tarek Fahmy and his lab have designed and developed an exciting novel form of drug delivery, wherein multiple treatments can be packaged together within a tiny case — a nanolipogel (nLG). Professor Fahmy’s lab, together with collaborators at the Yale School of Medicine, is now looking to use these tiny particles to target cancer cells from an entirely new angle.
Cancer Treatments: Advancements and Limitations
Scientists have long puzzled over approaches to attacking cancerous tumors. With cancers collectively accounting for the second-largest cause of death in the developed world, it has become apparent that traditional treatments such as surgery, radiation therapy, and chemotherapy are in many situations inadequate. For instance, large or metastasized tumors often cannot be completely removed via surgery. Additionally, radiation and chemotherapy are both double-edged swords, capable of killing both cancerous and healthy cells. Although cancers represent a diverse set of diseases, they share some common characteristics that may be exploited to find alternatives to blasting the body with radiation or cytotoxic chemicals, thereby avoiding the harsh side effects that accompany these conventional treatments.
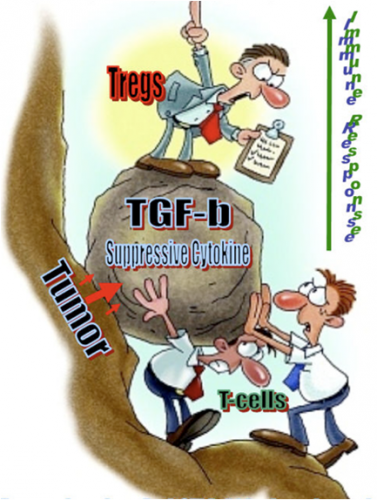
One such alternative is the emergent field of immunotherapy, which tries to empower the body’s own immune system to target and destroy malignant tumors. Previous research identified a cell signalling molecule called interleukin-2 (IL-2) as a candidate for use in immunotherapy, because it has been shown to activate natural killer (NK) cells and T cells in response to tumors, and specifically melanomas. Despite promising initial results, IL-2 therapy alone was ineffective in clinical trials, in part because the high doses required in order for it to be effective prove toxic to other healthy cells. But these studies revealed something ultimately more interesting: the cancer cells were cloaking themselves to evade detection and attack by the immune system. By secreting large quantities of transforming growth factor beta (TGF-β), the cancer cells could suppress the immune response around the tumor, thereby hampering the effectiveness of the attack. “That molecule,” as Professor Fahmy explains, “essentially cripples the immune system. When you have a lot of it around, you downregulate cytotoxic T-cells, downregulate antigen presentation. All that helps a tumor tolerate its environment.”
In order to sidestep this adaptation of cancer, researchers resort to a class of chemicals called TGF-β inhibitors. However, attempts to combine IL-2 and TGF-β inhibitors have largely failed because of basic kinetics; each drug has vastly different chemical properties which vary depending on how quickly each is distributed, absorbed, and broken down in the body. These fundamental processes directly impact how much of each drug actually reaches the tumor, thus limiting the therapeutic effectiveness of combination drug therapy. This ultimately leads to what is called the “problem of the pharmacokinetics of biodistribution.”
Nanolipogels: A New Approach to Cancer Immunotherapy
The idea of packaging drugs or other chemicals within guided nanoparticles is not a new one, but the scope of its applications is constantly being explored and expanded. Moreover, as increasingly sophisticated nanotechnological devices are developed, this process may be used to solve increasingly complex problems. While a few years ago, the major questions in the field were concerned with how nanoparticles could be guided to their target sites, research now has moved on to looking at how these particles can be adapted to specific applications. For example, currently in development are rapid diagnostic tests, which are facilitated by nanoparticles that brand or mark cancerous cells for detection or later excision.
Drawing upon his background in chemical engineering and immunology, Professor Fahmy saw an opportunity to resolve a biochemical issue using a technological solution. “Here you have two very different drugs, and you want to administer them into the same microenvironment. How do you do that? At that point, it really becomes an engineering problem.” In this light, the nLG technology developed by the Fahmy lab may be seen as the right tool for the job, as it is a combination of readily available parts which create a unique solution to an old problem.
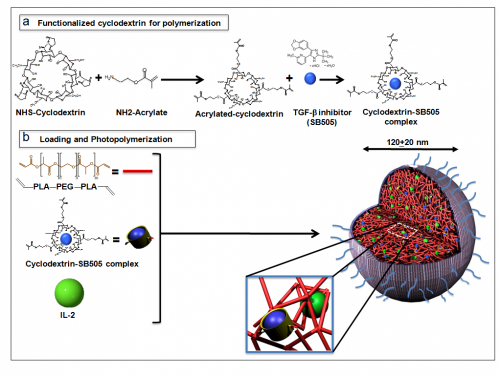
The process of piecing nLGs together begins with impregnating acrylated cyclodextrin (a biodegradable, biocompatible scaffolding compound) with TGF-β inhibitor molecules. Together with IL-2, these complexes are then entrapped within a biodegradable matrix of polylactic acid and polyethylene glycol (PLA—PEG). UV radiation is used to solidify the bonds of the matrix, and both drugs are thereby trapped within this core of the nLG. This core is then covered with a shell of phosphatidylcholine, another easily degradable polymer.
Although a seemingly straightforward process, each of these components must be carefully selected to ensure proper action in vivo. The separate outer shell has been demonstrated to degrade gradually within the body, yet still remain sturdy enough to carry the drugs to their destinations. Furthermore, the core matrix polymer degrades at a steady rate as well, so that high local dosages of both IL-2 and TGF-β inhibitor can be administered directly within the tumor microenvironment. What all the nLG components have in common is their ready biodegradability, which ensures that no harmful residues are left behind in the body after the period of therapy.

In many aggressive forms of melanoma and other cancers, rapidly growing tumors are accompanied by rapid blood vessel formation, leading to leaky vessels in the vasculature. When these nLGs are injected into the body, they are small enough to travel freely through the body’s bloodstream, but they have the ability to stick and clump preferentially to the tiny leaks within a tumor’s vessels, a form of “passive targeting.” Once localized to the tumor, these nLGs proceed to release their chemical payloads to simultaneously break down the cancerous cells’ defenses and activate the immune system to attack the malignant cells.

Application and Future Directions
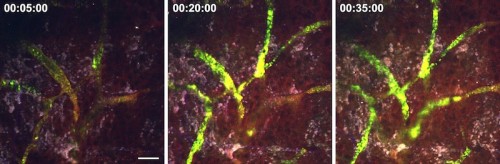
In animal trials, the Fahmy lab used their nLG system with a combination of IL-2 and TGF-β inhibitors in mice with melanomas and metastasized lung melanomas. When compared to therapies using the drugs alone, and the control group with no drugs at all, their nLG therapy significantly suppressed tumor growth and improved survival rates. Fluorescent imaging confirmed sustained release of both drugs in tumors as well as increased recruitment of T cells and NK cells. Although the early results appear promising, it is important to acknowledge that the technology is far from commercial use in human patients.
“The question that we need to focus on now,” says Professor Fahmy, “is how do you get from the proof-of-concept phase into use in humans for melanomas? Many drugs fall into what they call the valley of death; you develop something interesting in the lab, but you get this great chasm of empty space.” Without institutional and commercial support, many exciting potential therapies never make it to clinical testing.
The nLG technology is currently being investigated, in collaboration with commercial interests and the Yale Office of Cooperative Research, as a potential new drug in preclinical evaluation for human melanomas. The fact that this novel therapy uses already FDA-approved components could help accelerate progress towards widespread release, should clinical trials prove successful.

Although it has thus far been tested only as a melanoma treatment, the nLG platform can be adapted to accommodate a wide range of drugs. Consequently, as a drug delivery system, nLGs could potentially treat anything from autoimmune disorders to infectious diseases. Its mechanism of gradual, targeted release can help localize drugs that require high doses to be effective, while avoiding toxicity to other cells.
While there is still a long way to go to perfect these nanolipogels, Professor Fahmy believes that the time is near for science fiction to leap into the clinic. “There’s a lot of interest now in moving things into this new age of nanotechnology in drug delivery […] The people at Yale are very open-minded; they’re adventurous, they want to do new things. They recognize the limitations of current drugs and pharmaceuticals, and they recognize that technology is almost ripe.”
About the Author: Andrew Qi is a sophomore in Morse College studying Molecular Biochemistry and Biophysics, with a second interest in Biomedical Engineering.
Acknowledgements: The author would like to thank Professor Tarek Fahmy for his time and insight about work and life.
