Most people know that neurons die out with age, loosening our grip on reality. Generally, people also believe that neuron loss with age cannot be prevented.
But what if neuron loss over time was not an undeniable outcome of nature, and instead something that we could treat? A new study at the Yale School of Medicine by Professor Marc Hammarlund and postdoctoral fellow Alexandra Byrne suggests that neuron loss may not be correlated with age, but that it may be regulated by something else entirely.
Neurons, which transfer electrical signals between the brain and the body, have spindly projections called axons. Axons connect neurons and pass along signals from one nerve cell to another; thus, axon regeneration is a process integral to proper brain function: It allows the body to repair important neuronal connections after injury. As most organisms age, however, the potential for axons to regenerate declines.
To investigate the regulators of neuron loss, Hammarlund and Byrne examined the ability of animals to regenerate injured axons and how this ability declines with age. “The goal of our study was to identify genes that regulate this age-related decline in axon regeneration,” Byrne said. “More specifically, we wanted to characterize how these genes were controlling whether or not an aged axon could regenerate after injury.”
Instead of testing their hypothesis on human subjects, Hammarlund and Byrne used C. elegans, a one-millimeter-long transparent worm that researchers often use as a model organism due to its well-defined nervous system and transparent nature. Hammarlund had previously noted that these worms’ motor neurons, like those in humans, decline with aging. Because many genes in C. elegans are conserved in mammals, they function similarly to mammalian genes, making them good models. In addition, the worm’s nervous system is small and well-defined, making it much easier to study than that of a human.
As an adult animal ages, its neurons exhibit several decreased functions: retraction, growth response, and extension. Retraction, also known as pruning, is an important function of neurons that allows them to recycle axonal contents to different parts of the neuron. Growth response and extension are key functions that allow a neuron to respond to injury; when a break is detected, they help initiate neuron growth and extend the axon to bridge the gap of the break. Loss of these functions severely limits the ability of neurons to regenerate.
Hammarlund and Byrne investigated these particular aspects of neuronal aging. They began by researching the genetic relationship between age and axon regeneration. To do this, they injured neurons using a laser beam. They then watched the neurons regenerate, closely monitoring their progress to track what percentage of the axons destroyed by the laser beam ultimately regrew. This process was carried out in both “young,” one-day-old adult worms and “aged,” five-day-old adult worms to determine how much age affects regeneration. In the young adults, 65% of axons regenerated in response to injury, while in aged adults, only 28% of the axons regrew.
This regeneration difference was mainly due to the failure of growth cones, which are the extensions at the ends of growing axons that help determine the direction in which to initiate growth. In the axons that did begin regeneration, 31% in young adults and 12% in aged adults made substantial growth progress towards reconnecting with the dorsal nerve cord, which is similar to the spinal cord in mammals. With these results, Byrne and Hammarlund showed that when compared to those of younger adults, aged adults’ brains are less able to initiate and extend axon growth in response to injury.
After establishing that age was the factor that prevented neuron growth and regeneration, the researchers set out to find some way to delay neural aging. Byrne and Hammarlund hypothesized that certain genetic pathways play a role in physical aging and deterioration; manipulating these pathways might suppress the decline in axon regeneration. They tested several pathways to find one that, when altered, would produce aged adults that were physically as old as the control, but able to regenerate axons as quickly as young adults. In other words, the scientists were looking for something quite remarkable: a pathway they could manipulate to create an old worm with the brain of a young one.

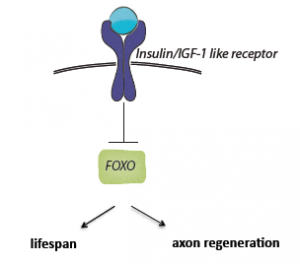
The pathway they discovered, called daf-2, is an insulin inhibiting pathway. Daf-2 is the only member of the insulin receptor family that exists in C. elegans and is known to regulate life span. When researchers in the Byrne and Hammarlund groups modified this pathway, ten-day-old adults demonstrated neural regeneration ability that far surpassed that of the control group.
These results imply that the daf-2 pathway regulates age and neuron regeneration separately. These two factors, contrary to intuition and prior belief, are completely independent: as the researchers showed, an animal can age at a normal rate but still have the neuron regeneration rate characteristic of a younger animal.
The newfound effects of this pathway were surprising. The daf-2 pathway was already known to regulate life span, but that it also regulates regeneration ability, independent of life span, is a recent insight. With this knowledge, Byrne and Hammarlund made their most exciting discovery. “By activating the insulin pathway specifically in neurons,” they wrote, “we were able to create old worms whose axons successfully regenerated despite the worms’ normal life spans. We were also able to do the opposite: create worms that lived extended lives, yet had axons that did not regenerate well.” They have shown that the decrease in axonal regeneration ability is not simply a consequence of old age, but rather that the insulin pathway is responsible for inhibiting regeneration with age. Their research demonstrates that the neuronal health span and life span can be uncoupled with different pathway regulation.
While this study is still in its preliminary phase, Hammarlund explained that its effects are far-reaching. “The study has significant implications for further research into how the nervous system regulates its response to time autonomously, and into the signaling pathways identified,” he said. The fact that the nervous system seems to be governed by a different regulation system than its age opens many doors. Researchers can further explore pathways that prevent nervous system deterioration as organisms get older. The knowledge that insulin regulates motor neuron regeneration furthers researchers’ understanding of regeneration response to injury and suggests additional pathways that can be explored.
Identifying genes that are involved in this regulation in aged animals increases our understanding of how the nervous system ages. We can control neuronal health with a mechanism separate from life span. The creation of genetically modified worms that regenerate neurons even at an older age suggests that such a distinct separation of age and neuron health may even be possible for humans in the future. The discovery of a similar neuron regeneration regulation pathway in humans may hold medical implications for Alzheimer’s disease or other neuronal degenerative diseases. Regulating the loss of neuron regeneration abilities can potentially prevent or treat diseases like these. Alzheimer’s affects 26.6 million people worldwide, so such a remarkable discovery would help improve many lives.
